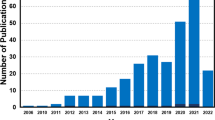
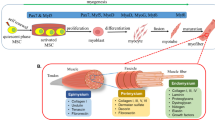
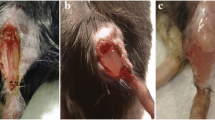
Abstract
Purpose of Review
Skeletal muscle tissue engineering is a field of vital importance to many sufferers of volumetric muscle loss (VML) and other muscular pathologies. Bioinductive scaffolds can host cells and modulate their behaviour and thus serve as a platform for muscle tissue growth.
Recent Findings
Scaffolds for skeletal muscle engineering can be composed of synthetic polymers, naturally derived polymers, decellularized extracellular matrix (ECM) or any combination of these. They lead to active tissue regeneration by modulating the initial inflammatory response, recruiting progenitor cells and determining cell phenotype. Their breakdown allows cellular migration while releasing stored stimulatory compounds.
Summary
Understanding the effects and advantages of the different biomaterial options might facilitate the design of novel scaffolds that support muscular regeneration, thereby restoring function and appearance to ailing patients. This review aims to present the latest advances in scaffold-based skeletal muscle tissue regeneration.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of applied physiology (Bethesda, Md: 1985). 2000;89(1):81–8.
Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–64.
Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med. 1986;14(4):303–8.
Ikemoto-Uezumi M, Uezumi A, Tsuchida K, Fukada S, Yamamoto H, Yamamoto N, et al. Pro-insulin-like growth factor-II ameliorates age-related inefficient regenerative response by orchestrating self-reinforcement mechanism of muscle regeneration. Stem cells (Dayton, Ohio). 2015;33(8):2456–68.
Ye F, Mathur S, Liu M, Borst SE, Walter GA, Sweeney HL, et al. Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol. 2013;98(5):1038–52.
Takahashi T, Ishida K, Itoh K, Konishi Y, Yagyu KI, Tominaga A, et al. IGF-I gene transfer by electroporation promotes regeneration in a muscle injury model. Gene Ther. 2003;10(8):612–20.
Armand AS, Launay T, Pariset C, Della Gaspera B, Charbonnier F, Chanoine C. Injection of FGF6 accelerates regeneration of the soleus muscle in adult mice. Biochim Biophys Acta. 2003;1642(1–2):97–105.
Hwang JH, Kim IG, Piao S, Jung AR, Lee JY, Park KD, et al. Combination therapy of human adipose-derived stem cells and basic fibroblast growth factor hydrogel in muscle regeneration. Biomaterials. 2013;34(25):6037–45.
Kelc R, Trapecar M, Gradisnik L, Rupnik MS, Vogrin M. Platelet-rich plasma, especially when combined with a TGF-beta inhibitor promotes proliferation, viability and myogenic differentiation of myoblasts in vitro. PLoS One. 2015;10(2):e0117302.
Chan YS, Li Y, Foster W, Fu FH, Huard J. The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med. 2005;33(1):43–51.
Fukushima K, Badlani N, Usas A, Riano F, Fu F, Huard J. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29(4):394–402.
Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science (New York, NY). 2005;309(5743):2064–7.
van Wachem PB, Brouwer LA, van Luyn MJ. Absence of muscle regeneration after implantation of a collagen matrix seeded with myoblasts. Biomaterials. 1999;20(5):419–26.
Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32(34):8905–14.
Borschel GH, Dow DE, Dennis RG, Brown DL. Tissue-engineered axially vascularized contractile skeletal muscle. Plast Reconstr Surg. 2006;117(7):2235–42.
Corona BT, Machingal MA, Criswell T, Vadhavkar M, Dannahower AC, Bergman C, et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng A. 2012;18(11–12):1213–28.
Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng A. 2011;17(17–18):2291–303.
Skuk D. Acute rejection of myofibers in nonhuman primates: key histopathologic features. J Neuropathol Exp Neurol. 2012;71(5):398–412.
Conconi MT, De Coppi P, Bellini S, Zara G, Sabatti M, Marzaro M, et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials. 2005;26(15):2567–74.
Park S, Choi Y, Jung N, Yu Y, Ryu KH, Kim HS, et al. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med. 2016;37(5):1209–20.
Fujita R, Tamai K, Aikawa E, Nimura K, Ishino S, Kikuchi Y, et al. Endogenous mesenchymal stromal cells in bone marrow are required to preserve muscle function in mdx mice. Stem cells (Dayton, Ohio). 2015;33(3):962–75.
Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–65.
Jeong J, Shin K, Lee SB, Lee DR, Kwon H. Patient-tailored application for Duchene muscular dystrophy on mdx mice based induced mesenchymal stem cells. Exp Mol Pathol. 2014;97(2):253–8.
von Roth P, Duda GN, Radojewski P, Preininger B, Perka C, Winkler T. Mesenchymal stem cell therapy following muscle trauma leads to improved muscular regeneration in both male and female rats. Gender medicine. 2012;9(2):129–36.
Merritt EK, Cannon MV, Hammers DW, Le LN, Gokhale R, Sarathy A, et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng A. 2010;16(9):2871–81.
Carnio S, Serena E, Rossi CA, De Coppi P, Elvassore N, Vitiello L. Three-dimensional porous scaffold allows long-term wild-type cell delivery in dystrophic muscle. J Tissue Eng Regen Med. 2011;5(1):1–10.
Oliva F, Via AG, Kiritsi O, Foti C, Maffulli N. Surgical repair of muscle laceration: biomechanical properties at 6 years follow-up. Muscles, ligaments and tendons journal. 2013;3(4):313–7.
Kragh JF Jr, Svoboda SJ, Wenke JC, Ward JA, Walters TJ. Epimysium and perimysium in suturing in skeletal muscle lacerations. J Trauma. 2005;59(1):209–12.
Menetrey J, Kasemkijwattana C, Fu FH, Moreland MS, Huard J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med. 1999;27(2):222–9.
Äärimaa V, Kääriäinen M, Vaittinen S, Tanner J, Järvinen T, Best T, et al. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul Disord. 2004;14(7):421–8.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–74.
Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries biology and treatment. Am J Sports Med. 2005;33(5):745–64.
Hurme T, Kalimo H, Lehto M, Jarvinen M. Healing of skeletal muscle injury: an ultrastructural and immunohistochemical study. Med Sci Sports Exerc. 1991;23(7):801–10.
Brigitte M, Schilte C, Plonquet A, Baba-Amer Y, Henri A, Charlier C, et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62(1):268–79.
Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–69.
Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18(3):482–96.
Badylak SF. Host response to biomaterials: the impact of host response on biomaterial selection. New York: Elsevier Science; 2015.
Molea G, Schonauer F, Bifulco G, D'Angelo D. Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (Polydioxanone, Poliglecaprone 25, Glycomer 631). Br J Plast Surg. 2000;53(2):137–41.
Lam CX, Savalani MM, Teoh S-H, Hutmacher DW. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: accelerated versus simulated physiological conditions. Biomed Mater. 2008;3(3):034108.
Williamson MR, Adams EF, Coombes AGA. Gravity spun polycaprolactone fibres for soft tissue engineering: interaction with fibroblasts and myoblasts in cell culture. Biomaterials. 2006;27(7):1019–26.
McKeon-Fischer K, Flagg D, Freeman J. Coaxial electrospun poly (ε-caprolactone), multiwalled carbon nanotubes, and polyacrylic acid/polyvinyl alcohol scaffold for skeletal muscle tissue engineering. J Biomed Mater Res A. 2011;99(3):493–9.
Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE engineering in medicine and biology magazine: the quarterly magazine of the Engineering in Medicine & Biology Society. 2008;27(5):109–13.
• Zhao W, Ju YM, Christ G, Atala A, Yoo JJ, Lee SJ. Diaphragmatic muscle reconstruction with an aligned electrospun poly(ε-caprolactone)/collagen hybrid scaffold. Biomaterials. 2013;34(33):8235–40. This study showed positive scaffold-mediated muscle regeneration in a model with clear clinical translatability. It provided a good example of a composite natural/synthetic polymer.
Zambon JP, de Sa Barretto LS, Nakamura AN, Duailibi S, Leite K, Magalhaes RS, et al. Histological changes induced by Polyglycolic-Acid (PGA) scaffolds seeded with autologous adipose or muscle-derived stem cells when implanted on rabbit bladder. Organ. 2014;10(2):278–88.
Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42(3):396–402.
Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60(4):668–78.
Yang HS, Ieronimakis N, Tsui JH, Kim HN, Suh K-Y, Reyes M, et al. Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy. Biomaterials. 2014;35(5):1478–86.
Wang P-Y, Wu T-H, Tsai W-B, Kuo W-H, Wang M-J. Grooved PLGA films incorporated with RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloids Surf B: Biointerfaces. 2013;110:88–95.
Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, Chuah M, et al. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29(1):75–84.
• Manchineella S, Thrivikraman G, Khanum KK, Ramamurthy PC, Basu B, Govindaraju T. Pigmented silk nanofibrous composite for skeletal muscle tissue engineering. Advanced healthcare materials. 2016;5(10):1222–32. The authors concentrated on the electroactive properties of MPCs when developing the scaffold described in this study. They characterized its electrical properties and demonstrated its in vitro myogenic capabilities.
•• Shin YC, Lee JH, Jin L, Kim MJ, Kim YJ, Hyun JK, et al. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices. Journal of nanobiotechnology. 2015;13:21. This study is an impressive example of an advanced biomaterial, formed from a composite of PLGA with collagen and graphene, with potential for skeletal muscle tissue engineering.
Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278(17):14587–90.
Chiron S, Tomczak C, Duperray A, Laine J, Bonne G, Eder A, et al. Complex interactions between human myoblasts and the surrounding 3D fibrin-based matrix. PLoS One. 2012;7(4):e36173.
Rybalko VY, Pham CB, Hsieh PL, Hammers DW, Merscham-Banda M, Suggs LJ, et al. Controlled delivery of SDF-1alpha and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomaterials science. 2015;3(11):1475–86.
Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277(6):4223–31.
Kroehne V, Heschel I, Schugner F, Lasrich D, Bartsch JW, Jockusch H. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med. 2008;12(5a):1640–8.
Dai W, Hale SL, Kay GL, Jyrala AJ, Kloner RA. Delivering stem cells to the heart in a collagen matrix reduces relocation of cells to other organs as assessed by nanoparticle technology. Regen Med. 2009;4(3):387–95.
Smith AS, Passey SL, Martin NR, Player DJ, Mudera V, Greensmith L, et al. Creating interactions between tissue-engineered skeletal muscle and the peripheral nervous system. Cells Tissues Organs. 2016;202(3–4):143–58.
Boyd A, Chakrabarty AM. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J Ind Microbiol. 1995;15(3):162–8.
Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53.
Stilhano RS, Madrigal JL, Wong K, Williams PA, Martin PK, Yamaguchi FS, et al. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. Journal of controlled release : official journal of the Controlled Release Society. 2016;237:42–9.
Raya B, Caroline C, Daniel C, Benjamin D, Christophe T, Philippe B, et al. Elaboration and evaluation of alginate foam scaffolds for soft tissue engineering. Int J Pharm. 2017;524(1):433–42.
Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12(5):1295–304.
Pumberger M, Qazi TH, Ehrentraut MC, Textor M, Kueper J, Stoltenburg-Didinger G, et al. Synthetic niche to modulate regenerative potential of MSCs and enhance skeletal muscle regeneration. Biomaterials. 2016;99:95–108.
Stilhano RS, Madrigal JL, Wong K, Williams PA, Martin PKM, Yamaguchi FSM, et al. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. J Control Release. 2016;237:42–9.
Wang W, Fan M, Zhang L, Liu SH, Sun L, Wang CY. Compatibility of hyaluronic acid hydrogel and skeletal muscle myoblasts. Biomedical materials (Bristol, England). 2009;4(2):025011.
Rossi CA, Flaibani M, Blaauw B, Pozzobon M, Figallo E, Reggiani C, et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011;25(7):2296–304.
Desiderio V, De Francesco F, Schiraldi C, De Rosa A, La Gatta A, Paino F, et al. Human Ng2+ adipose stem cells loaded in vivo on a new crosslinked hyaluronic acid-lys scaffold fabricate a skeletal muscle tissue. J Cell Physiol. 2013;228(8):1762–73.
Kong WH, Sung DK, Kim H, Yang J-A, Ieronimakis N, Kim KS, et al. Self-adjuvanted hyaluronate—antigenic peptide conjugate for transdermal treatment of muscular dystrophy. Biomaterials. 2016;81:93–103.
Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell–ECM interactions to tissue engineering. J Cell Physiol. 2004;199(2):174–80.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods (San Diego, Calif). 2015;84:25–34.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness. J Cell Biol. 2004;166(6):877–87.
Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci. 2004;117(1):73–84.
Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol. 2004;268(2):358–71.
El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258(2):279–87.
Lewis M, Tippett H, Sinanan A, Morgan M, Hunt N. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. Journal of Muscle Research & Cell Motility. 2000;21(3):223–33.
Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J. Proteolytic control of growth factor availability. APMIS. 1999;107(1–6):80–5.
Brennan EP, Tang X-H, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2(8):491.
Agrawal V, Kelly J, Tottey S, Daly KA, Johnson SA, Siu BF, et al. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Engineering - Part A. 2011;17(23–24):3033–44.
Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng A. 2010;17(1–2):37–44.
Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–5.
Mannello F, Tonti GA, Bagnara GP, Papa S. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem cells (Dayton, Ohio). 2006;24(3):475–81.
Porzionato A, Sfriso MM, Pontini A, Macchi V, Petrelli L, Pavan PG, et al. Decellularized human skeletal muscle as biologic scaffold for reconstructive surgery. Int J Mol Sci. 2015;16(7):14808–31.
Wilson K, Terlouw A, Roberts K, Wolchok JC. The characterization of decellularized human skeletal muscle as a blueprint for mimetic scaffolds. Journal of materials science Materials in medicine. 2016;27(8):125.
Sicari BM, Agrawal V, Siu BF, Medberry CJ, Dearth CL, Turner NJ, et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng A. 2012;18(19–20):1941–8.
Chen XK, Walters TJ. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2013;66(12):1750–8.
Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30(12):2393–9.
Turner NJ, Yates AJ Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng A. 2010;16(11):3309–17.
Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31(29):7475–84.
Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg. 2006;88(12):2673–86.
McClelland R, Wauthier E, Uronis J, Reid L. Gradients in the liver’s extracellular matrix chemistry from periportal to pericentral zones: influence on human hepatic progenitors. Tissue Eng A. 2008;14(1):59–70.
Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, et al. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009;30(23):4021–8.
Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng A. 2009;15(2):231–41.
• Keane TJ, DeWard A, Londono R, Saldin LT, Castleton AA, Carey L, et al. Tissue-specific effects of esophageal extracellular matrix. Tissue Eng A. 2015;21(17–18):2293–300. The authors of this study convincingly demonstrated the advantages of site-specific ECM for in vitro differentiation, maturation and proliferation of stem cells. Translation of these properties to an in vivo setting would be a big boost to the field.
Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33(10):2916–25.
Kasukonis B, Kim J, Brown L, Jones J, Ahmadi S, Washington T, et al. Codelivery of infusion decellularized skeletal muscle with minced muscle autografts improved recovery from volumetric muscle loss injury in a rat model. Tissue Eng A. 2016;22(19–20):1151–63.
Corona BT, Garg K, Ward CL, McDaniel JS, Walters TJ, Rathbone CR. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. Am J Phys Cell Phys. 2013;305(7):C761–75.
Knappe S, Zammit P, Knight R. A population of Pax7-expressing muscle progenitor cells show differential responses to muscle injury dependent on developmental stage and injury extent. Front Aging Neurosci. 2015;7:161.
Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT. Ontogeny of expression of transforming growth factor-beta 1 (TGF-beta 1), TGF-beta 3, and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg. 2001;107(7):1787–94. discussion 1795-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Wound Healing and Tissue Repair
Rights and permissions
About this article
Cite this article
Iyer, H., Galiano, R.D. Bioinductive Scaffolds—Powerhouses of Skeletal Muscle Tissue Engineering. Curr Pathobiol Rep 5, 279–288 (2017). https://doi.org/10.1007/s40139-017-0151-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-017-0151-9