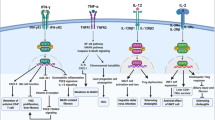
Abstract
Interleukin-22 (IL-22) belongs to IL-10 cytokine family which was initially described as a T helper 1 (Th1) and 17 (Th17) cytokine. Subsequently, numerous other sources were identified. The liver is often described as an immunological organ due to its particular contain in a large spectrum of immune cells of which certain subsets produce IL-22. In liver, expression of IL-22 receptor subunit-1 (IL-22R1), is restricted to hepatocytes, hepatic stellate cells, and liver progenitor cells which are involved in several hepatic diseases. IL-22 is known to play protective roles mainly through signal transducer of activator of transcription-3 (STAT3)-dependant signaling pathways. However, possible deleterious effects of IL-22 at specific stages along with the progression of the diseases and according to the etiology were reported. This review summarizes the current understandings of both beneficial and deleterious facets of IL-22 in liver diseases and discusses its putative targeting as a strategy for further clinical trials.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dumoutier L, Louahed J, Renauld J-C (2000) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164:1814–1819
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168:5397–5402
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133
Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR (2007) IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8:967–974
Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C (2006) Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res 16:902–907
Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT (2012) Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37:1061–1075
Mabuchi T, Takekoshi T, Hwang ST (2011) Epidermal CCR6+ γδ T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol 187:5026–5031
Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, Dungan LS, Sutton CE, Streubel G (2013) Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med 210:1117–1124
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE (2013) Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149
Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T (2009) Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol 254:81–84
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10:857–863
Vantourout P, Hayday A (2013) Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 13:88–100
Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, Fu YX (2014) Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40:25–39
Hansson M, Silverpil E, Lindén A, Glader P (2013) Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm Res 62:561–569
Zindl CL, Lai J-F, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT (2013) IL-22–producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci 110:12768–12773
Kulkarni O (2014) TLR4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol 25(5):978–989
Dudakov JA, Hanash AM, van den Brink MR (2015) Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33:747–785
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21:241–254
• Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin‐22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology 2012;56:1150–9. Outlines the molecular and cellular mechanims of IL-22 anti-fibrotic properties in liver
•• Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, Gershwin ME, Gao B. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology 2012;143:188–8.e187. This article uncovered mitogenic properties of IL-22 on liver stem/progenitor cell niche
Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld J-C (2002) Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line Pathways that are shared with and distinct from IL-10. J Biol Chem 277:33676–33682
Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM (2007) Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448:480–483
Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q (2009) Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30:576–587
Radaeva S, Sun R (2004) Pan Hn, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39:1332–1342
Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B (2011) Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 54:900–909
Murano T, Okamoto R, Ito G, Nakata T, Hibiya S, Shimizu H, Fujii S, Kano Y, Mizutani T, Yui S (2014) Hes1 promotes the IL-22-mediated antimicrobial response by enhancing STAT3-dependent transcription in human intestinal epithelial cells. Biochem Biophys Res Commun 443:840–846
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648–651
Mitra A, Raychaudhuri SK, Raychaudhuri SP (2012) IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 60:38–42
Cho K-A, Kim J-Y, Woo S-Y, Park HJ, Lee KH, Pae C-U (2012) Interleukin-17 and interleukin-22 induced proinflammatory cytokine production in keratinocytes via inhibitor of nuclear factor κB kinase-α expression. Ann Dermatol 24:398–405
Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S (2001) Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol 166:7096–7103
Logsdon NJ, Jones BC, Josephson K, Cook J, Walter MR (2002) Comparison of interleukin-22 and interleukin-10 soluble receptor complexes. J Interf Cytokine Res 22:1099–1112
Sabat R, Ouyang W, Wolk K (2014) Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 13:21–38
Ren X, Hu B, Colletti LM (2010) IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol 298:G74–G80
Chestovich PJ, Uchida Y, Chang W, Ajalat M, Lassman C, Sabat R, Busuttil RW, Kupiec-Weglinski JW (2012) IL-22: implications for liver ischemia/reperfusion injury. Transplantation 93:485
Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS (2011) In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology 54:252–261
Pan H, Hong F, Radaeva S, Gao B (2004) Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol 1:43–49
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27:647–659
Hernandez-Gea V, Friedman SL (2011) Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA (2014) Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14:181–194
Jeong WI, Park O, Radaeva S, Gao B (2006) STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology 44:1441–1451
• Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M. Osterreicher CH, Stickel F, Ley K, Brenner DA, Kisseleva. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012;143:765–76.e763. Shows a protective role of IL-22 against bile duct-ligation-induced hepatic fibrosis
Lu D-H, Guo X-Y, Qin S-Y, Luo W, Huang X-L, Chen M, Wang J-X, Ma S-J, Yang X-W, Jiang H-X (2015) Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol 21:1531
Wu LY, Liu S, Liu Y, Guo C, Li H, Li W, Jin X, Zhang K, Zhao P, Wei L, Zhao J (2015) Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol 158:77–87
Wynn TA (2004) Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol 4:583–594
Ge J, Wang K, Meng Q-H, Qi Z-X, Meng F-L, Fan Y-C (2010) Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 30:60–67
Gao B, Waisman A (2012) Th17 cells regulate liver fibrosis by targeting multiple cell types: many birds with one stone. Gastroenterology 143:536–539
Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, Jin L, Zhou C, Fu J, Gao B (2014) Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 59:1331–1342
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Investig 119:3573
Foster RG, Golden-Mason L, Rutebemberwa A, Rosen HR (2012) Interleukin (IL)-17/IL-22-producing T cells enriched within the liver of patients with chronic hepatitis C viral (HCV) infection. Dig Dis Sci 57:381–389
Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, Robek MD (2011) A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology 141:1897–1906
Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, Li Y, Park O, Dooley S, Ju C (2014) Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol 193:2512–2518
Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B (2010) Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52:1291–1300
Xing W-W, Zou M-J, Liu S, Xu T, Wang J-X, Xu D-G (2011) Interleukin-22 protects against acute alcohol-induced hepatotoxicity in mice. Biosci Biotechnol Biochem 75:1290–1294
Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z (2010) Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol 53:339–347
Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, Yin S, Fu XY, Gershwin ME, Lian ZX, Gao B (2009) Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology 137(2125–2135):e2121–2122
Rountree CB, Mishra L, Willenbring H (2012) Stem cells in liver diseases and cancer: recent advances on the path to new therapies. Hepatology 55:298–306
Tanaka M, Itoh T, Tanimizu N, Miyajima A (2011) Liver stem/progenitor cells: their characteristics and regulatory mechanisms. J Biochem 149:231–239
Michalopoulos GK (2011) Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol 43:173–179
Roskams T, Katoonizadeh A, Komuta M (2010) Hepatic progenitor cells: an update. Clin Liver Dis 14:705–718
He G, Yu G-Y, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M (2010) Hepatocyte IKKβ/NF-κB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17:286–297
Curd LM, Favors SE, Gregg RK (2012) Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol 168:192–199
Tang Y, Kitisin K, Jogunoori W, Li C, Deng C-X, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS (2008) Progenitor/stem cells give rise to liver cancer due to aberrant TGF-β and IL-6 signaling. Proc Natl Acad Sci 105:2445–2450
Yang W, Yan H-X, Chen L, Liu Q, He Y-Q, Yu L-X, Zhang S-H, Huang D-D, Tang L, Kong X-N (2008) Wnt/β-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 68:4287–4295
Ma S, Chan KW, Hu L, Lee TKW, Wo JYH, Ng IOL, Zheng BJ, Guan XY (2007) Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology 132:2542–2556
• Wang C, Yang W, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY. Hepatitis B virus X (HBx) induces tumorigenicity of hepatic progenitor cells in 3, 5‐diethoxycarbonyl‐1, 4‐dihydrocollidine‐treated HBx transgenic mice. Hepatology 2012;55:108–20. Liver progenitor cells exhibit ability to give rise of tumors in HBV infection
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Cytokines That Affect Liver Fibrosis and Activation of Hepatic Myofibroblasts.
Rights and permissions
About this article
Cite this article
Kong, X., Liu, W., Xia, Q. et al. Physiological and Pathological Properties of Interleukin-22 in Liver Diseases. Curr Pathobiol Rep 3, 307–313 (2015). https://doi.org/10.1007/s40139-015-0088-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-015-0088-9