Abstract
Purpose of Review
We discuss indications, contraindications, and outcomes, while addressing unique considerations for the use of extracorporeal membrane oxygenation (ECMO) in pediatric patients with cardiac disease.
Recent Findings
Increasing clinical experience and research in ECMO utilization for pediatric patients with cardiac disease have led to better understanding of the role of timing of postoperative cannulation, strategies for single ventricle anatomy, left heart decompression, management of anticoagulation, the role of venovenous ECMO, and long-term outcomes and follow-up in this patient population.
Summary
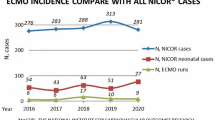
The use of ECMO for congenital and acquired heart disease has increased over time, with improving survival to hospital discharge. As more patients are cannulated onto ECMO, focus must shift towards optimizing management to further improve morbidity and mortality. Increased survival to discharge also requires that patients have structured follow-up to help maximize long term neurodevelopmental outcomes and quality of life post-critical illness with ECMO support.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59(3):202–10.
Brunetti MA, Gaynor JW, Retzloff LB, Lehrich JL, Banerjee M, Amula V, et al. Characteristics, risk factors, and outcomes of extracorporeal membrane oxygenation use in pediatric cardiac ICUs: a report from the Pediatric Cardiac Critical Care Consortium Registry. Pediatr Crit Care Med. 2018;19(6):544–52. https://doi.org/10.1097/PCC.0000000000001571.
Bembea MM, Ng DK, Rizkalla N, Rycus P, Lasa JJ, Dalton H, et al. Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in-hospital cardiac arrest: a report from the Get With the Guidelines-Resuscitation and the Extracorporeal Life Support Organization Registries. Crit Care Med. 2019;47(4):e278–e85. https://doi.org/10.1097/CCM.0000000000003622.
Duff JP, Topjian AA, Berg MD, Chan M, Haskell SE, Joyner BL, et al. 2019 American Heart Association focused update on pediatric advanced life support: an update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019;140(24):e904–e14. https://doi.org/10.1161/CIR.0000000000000731.
Marino BS, Tabbutt S, MacLaren G, Hazinski MF, Adatia I, Atkins DL, et al. Cardiopulmonary resuscitation in infants and children with cardiac disease: a scientific statement from the American Heart Association. Circulation. 2018;137(22):e691–782. https://doi.org/10.1161/CIR.0000000000000524.
Melvan JN, Davis J, Heard M, Trivedi JR, Wolf M, Kanter KR, et al. Factors associated with survival following extracorporeal cardiopulmonary resuscitation in children. World J Pediatr Congenit Heart Surg. 2020;11(3):265–74. https://doi.org/10.1177/2150135120902102.
Bautista-Hernandez V, Thiagarajan RR, Fynn-Thompson F, Rajagopal SK, Nento DE, Yarlagadda V, et al. Preoperative extracorporeal membrane oxygenation as a bridge to cardiac surgery in children with congenital heart disease. Ann Thorac Surg. 2009;88(4):1306–11. https://doi.org/10.1016/j.athoracsur.2009.06.074.
Ergün S, Yildiz O, Güneş M, Akdeniz HS, Öztürk E, Onan İ, et al. Use of extracorporeal membrane oxygenation in postcardiotomy pediatric patients: parameters affecting survival. Perfusion. 2020;35(7):608–20. https://doi.org/10.1177/0267659119897746.
Mascio CE, Austin EH, Jacobs JP, Jacobs ML, Wallace AS, He X, et al. Perioperative mechanical circulatory support in children: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2014;147(2):658–64: discussion 64-5. https://doi.org/10.1016/j.jtcvs.2013.09.075.
Cho HJ, Choi I, Kwak Y, Kim DW, Habimana R, Jeong IS. The outcome of post-cardiotomy extracorporeal membrane oxygenation in neonates and pediatric patients: a systematic review and meta-analysis. Front Pediatr. 2022;10:869283. https://doi.org/10.3389/fped.2022.869283.
Roeleveld PP, Mendonca M. Neonatal cardiac ECMO in 2019 and beyond. Front Pediatr. 2019;7:327. https://doi.org/10.3389/fped.2019.00327.
Gupta P, DasGupta R, Best D, Chu CB, Elsalloukh H, Gossett JM, et al. Delayed extracorporeal membrane oxygenation in children after cardiac surgery: two-institution experience. Cardiol Young. 2015;25(2):248–54. https://doi.org/10.1017/S1047951113002011.
Jaggers JJ, Forbess JM, Shah AS, Meliones JN, Kirshbom PM, Miller CE, et al. Extracorporeal membrane oxygenation for infant postcardiotomy support: significance of shunt management. Ann Thorac Surg. 2000;69(5):1476–83. https://doi.org/10.1016/s0003-4975(00)01330-8.
Ford MA, Gauvreau K, McMullan DM, Almodovar MC, Cooper DS, Rycus PT, et al. Factors associated with mortality in neonates requiring extracorporeal membrane oxygenation for cardiac indications: analysis of the Extracorporeal Life Support Organization Registry Data. Pediatr Crit Care Med. 2016;17(9):860–70. https://doi.org/10.1097/PCC.0000000000000842.
Alten J, Cooper DS, Klugman D, Raymond TT, Wooton S, Garza J, et al. Preventing cardiac arrest in the pediatric cardiac intensive care unit through multicenter collaboration. JAMA Pediatr. 2022;176(10):1027–36. https://doi.org/10.1001/jamapediatrics.2022.2238. The implementation of a low-technology cardiac arrest prevention practice bundle activated in 2664 of 10,510 hospital admissions led to significant in-hospital cardiac arrest reduction across 15 pediatric cardiac intensive care units.
Sperotto F, Polito A, Amigoni A, Maschietto N, Thiagarajan RR. Left atrial decompression in pediatric patients supported with extracorporeal membrane oxygenation for failure to wean from cardiopulmonary bypass: a propensity-weighted analysis. J Am Heart Assoc. 2022;11(23):e023963. https://doi.org/10.1161/JAHA.121.023963.
Agarwal HS, Hardison DC, Saville BR, Donahue BS, Lamb FS, Bichell DP, et al. Residual lesions in postoperative pediatric cardiac surgery patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg. 2014;147(1):434–41. https://doi.org/10.1016/j.jtcvs.2013.03.021.
Howard TS, Kalish BT, Wigmore D, Nathan M, Kulik TJ, Kaza AK, et al. Association of extracorporeal membrane oxygenation support adequacy and residual lesions with outcomes in neonates supported after cardiac surgery. Pediatr Crit Care Med. 2016;17(11):1045–54. https://doi.org/10.1097/PCC.0000000000000943.
Kato A, Lo Rito M, Lee KJ, Haller C, Guerguerian AM, Sivarajan VB, et al. Impacts of early cardiac catheterization for children with congenital heart disease supported by extracorporeal membrane oxygenation. Catheter Cardiovasc Interv. 2017;89(5):898–905. https://doi.org/10.1002/ccd.26632.
Panda BR, Alphonso N, Govindasamy M, Anderson B, Stocker C, Karl TR. Cardiac catheter procedures during extracorporeal life support: a risk-benefit analysis. World J Pediatr Congenit Heart Surg. 2014;5(1):31–7. https://doi.org/10.1177/2150135113505297.
Allan CK, Thiagarajan RR, del Nido PJ, Roth SJ, Almodovar MC, Laussen PC. Indication for initiation of mechanical circulatory support impacts survival of infants with shunted single-ventricle circulation supported with extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2007;133(3):660–7. https://doi.org/10.1016/j.jtcvs.2006.11.013.
Grant S, Faraoni D, DiNardo J, Odegard K. Predictors of mortality in children with pulmonary atresia with intact ventricular septum. Pediatr Cardiol. 2017;38(8):1627–32. https://doi.org/10.1007/s00246-017-1706-6.
Amodeo A, Stojanovic M, Dave H, Cesnjevar R, Konetzka A, Erdil T, et al. Bridging with veno-arterial extracorporeal membrane oxygenation in children: a 10-year single-center experience. Life. 2022;12(9) https://doi.org/10.3390/life12091398.
Alsoufi B, Wolf M, Botha P, Kogon B, McCracken C, Ehrlich A, et al. Late outcomes of infants supported by extracorporeal membrane oxygenation following the Norwood operation. World J Pediatr Congenit Heart Surg. 2015;6(1):9–17. https://doi.org/10.1177/2150135114558072.
Debrunner MG, Porayette P, Breinholt JP, Turrentine MW, Cordes TM. Midterm survival of infants requiring postoperative extracorporeal membrane oxygenation after Norwood palliation. Pediatr Cardiol. 2013;34(3):570–5. https://doi.org/10.1007/s00246-012-0499-x.
Friedland-Little JM, Aiyagari R, Yu S, Donohue JE, Hirsch-Romano JC. Survival through staged palliation: fate of infants supported by extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg. 2014;97(2):659–65. https://doi.org/10.1016/j.athoracsur.2013.10.066.
Mayr B, Kido T, Holder S, Wallner M, Vodiskar J, Strbad M, et al. Single-centre outcome of extracorporeal membrane oxygenation after the neonatal Norwood procedure. Eur J Cardiothorac Surg. 2022;62(3) https://doi.org/10.1093/ejcts/ezac129.
Mitchell EA, Gomez D, Joy BF, Fernandez RP, Cheatham JP, Galantowicz M, et al. ECMO: incidence and outcomes of patients undergoing the hybrid procedure. Congenit Heart Dis. 2016;11(2):169–74. https://doi.org/10.1111/chd.12311.
Ravishankar C, Dominguez TE, Kreutzer J, Wernovsky G, Marino BS, Godinez R, et al. Extracorporeal membrane oxygenation after stage I reconstruction for hypoplastic left heart syndrome. Pediatr Crit Care Med. 2006;7(4):319–23. https://doi.org/10.1097/01.PCC.0000227109.82323.CE.
Roeleveld PP, Wilde R, Hazekamp M, Rycus PT, Thiagarajan RR. Extracorporeal membrane oxygenation in single ventricle lesions palliated via the hybrid approach. World J Pediatr Congenit Heart Surg. 2014;5(3):393–7. https://doi.org/10.1177/2150135114526420.
Sherwin ED, Gauvreau K, Scheurer MA, Rycus PT, Salvin JW, Almodovar MC, et al. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2012;144(6):1337–43. https://doi.org/10.1016/j.jtcvs.2012.03.035.
Egan MJ, Hill SL, Boettner BL, Holzer RJ, Phillips AB, Galantowicz M, et al. Predictors of retrograde aortic arch obstruction after hybrid palliation of hypoplastic left heart syndrome. Pediatr Cardiol. 2011;32(1):67–75. https://doi.org/10.1007/s00246-010-9820-8.
Stoica SC, Philips AB, Egan M, Rodeman R, Chisolm J, Hill S, et al. The retrograde aortic arch in the hybrid approach to hypoplastic left heart syndrome. Ann Thorac Surg. 2009;88(6):1939–46; discussion 46-7. https://doi.org/10.1016/j.athoracsur.2009.06.115.
Fernandez RP, Joy BF, Allen R, Stewart J, Miller-Tate H, Miao Y, et al. Interstage survival for patients with hypoplastic left heart syndrome after ECMO. Pediatr Cardiol. 2017;38(1):50–5. https://doi.org/10.1007/s00246-016-1483-7.
Botha P, Deshpande SR, Wolf M, Heard M, Alsoufi B, Kogon B, et al. Extracorporeal membrane oxygenator support in infants with systemic-pulmonary shunts. J Thorac Cardiovasc Surg. 2016;152(3):912–8. https://doi.org/10.1016/j.jtcvs.2016.03.075.
Brown G, Moynihan KM, Deatrick KB, Hoskote A, Sandhu HS, Aganga D, et al. Extracorporeal Life Support Organization (ELSO): guidelines for pediatric cardiac failure. ASAIO J. 2021;67(5):463–75. https://doi.org/10.1097/MAT.0000000000001431.
Booth KL, Roth SJ, Thiagarajan RR, Almodovar MC, del Nido PJ, Laussen PC. Extracorporeal membrane oxygenation support of the Fontan and bidirectional Glenn circulations. Ann Thorac Surg. 2004;77(4):1341–8. https://doi.org/10.1016/j.athoracsur.2003.09.042.
Gomez D, Duffy V, Hersey D, Backes C, Rycus P, McConnell P, et al. Extracorporeal membrane oxygenation outcomes after the comprehensive stage II procedure in patients with single ventricles. Artif Organs. 2017;41(1):66–70. https://doi.org/10.1111/aor.12810.
Jolley M, Thiagarajan RR, Barrett CS, Salvin JW, Cooper DS, Rycus PT, et al. Extracorporeal membrane oxygenation in patients undergoing superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2014;148(4):1512–8. https://doi.org/10.1016/j.jtcvs.2014.04.028.
Schubert S, Opgen-Rhein B, Boehne M, Weigelt A, Wagner R, Müller G, et al. Severe heart failure and the need for mechanical circulatory support and heart transplantation in pediatric patients with myocarditis: Results from the prospective multicenter registry "MYKKE". Pediatr Transplant. 2019;23(7):e13548. https://doi.org/10.1111/petr.13548.
Teele SA, Allan CK, Laussen PC, Newburger JW, Gauvreau K, Thiagarajan RR. Management and outcomes in pediatric patients presenting with acute fulminant myocarditis. J Pediatr. 2011;158(4):638–43.e1. https://doi.org/10.1016/j.jpeds.2010.10.015.
Gutierrez ME, Anders M, Guffey D, Denfield SW, Deshpande SR, Rajagopal SK, et al. Extracorporeal membrane oxygenation cannulation timing in the pediatric myocarditis population: an exploratory analysis from the Extracorporeal Life Support Organization Registry. Crit Care Explor. 2023;5(1):e0826. https://doi.org/10.1097/CCE.0000000000000826. An analysis of Extracorporeal Life Support Organization registry data from patients ≤18 years who received ECMO support for myocarditis between 2007-2018 suggested that patients approaching ECMO cannulation who have not experienced cardiac arrest may have improved survival if cannulated onto ECMO early after intubation.
Choudhury TA, Ofori-Amanfo G, Choi J, Eisenberg RE, Rycus P, Medar SS, et al. Left heart decompression on veno-arterial extracorporeal membrane oxygenation in children with dilated cardiomyopathy and myocarditis: an Extracorporeal Life Support Organization Registry review. Pediatr Crit Care Med. 2021;22(12):1026–32. https://doi.org/10.1097/PCC.0000000000002775.
Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63(3):257–65. https://doi.org/10.1097/MAT.0000000000000553.
Weber C, Deppe AC, Sabashnikov A, Slottosch I, Kuhn E, Eghbalzadeh K, et al. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion. 2018;33(4):283–8. https://doi.org/10.1177/0267659117745369.
Zampi JD, Alghanem F, Yu S, Callahan R, Curzon CL, Delaney JW, et al. Relationship between time to left atrial decompression and outcomes in patients receiving venoarterial extracorporeal membrane oxygenation support: a multicenter pediatric interventional cardiology early-career society study. Pediatr Crit Care Med. 2019;20(8):728–36. https://doi.org/10.1097/PCC.0000000000001936.
Extracorporeal life support. The ELSO red book. 6th ed. Ann Arbor, Michigan: Extracorporeal Life Support Organization; 2022.
Bembea MM, Loftis LL, Thiagarajan RR, Young CC, McCadden TP, Newhams MM, et al. Extracorporeal membrane oxygenation characteristics and outcomes in children and adolescents with COVID-19 or multisystem inflammatory syndrome admitted to U.S. ICUs. Pediatr Crit Care Med. 2023; https://doi.org/10.1097/PCC.0000000000003212. Among patients <21 years admitted to intensive care units in 63 hospitals from 32 U.S. states reporting to the Overcoming COVID-19 public health surveillance registry between March 2020 and December 2021, 37 of 1,530 (2.4%) of patients with multisystem inflammatory syndrome in children (MIS-C) and 71 of 1,203 (5.9%) of patients with acute COVID-19 were supported on ECMO. Patients on ECMO with MIS-C versus COVID-19 were supported more often with venoarterial ECMO (92% vs 41%) for primary cardiac indications (87% vs 23%), had ECMO initiated earlier (median 1 vs 5 d from hospitalization), shorter ECMO courses (median 3.9 vs 14 d), shorter hospital length of stay (median 20 vs 52 d), lower in-hospital mortality (27% vs 37%), and less major morbidity at discharge in survivors
Di Nardo M, Hoskote A, Thiruchelvam T, Lillie J, Horan M, Belda Hofheinz S, et al. Extracorporeal membrane oxygenation in children with coronavirus disease 2019: preliminary report from the Collaborative European Chapter of the Extracorporeal Life Support Organization Prospective Survey. ASAIO J. 2021;67(2):121–4. https://doi.org/10.1097/MAT.0000000000001309.
Di Nardo M, De Piero ME, Hoskote A, Belohlavek J, Lorusso R. Committee EnapC-WGaES. Extracorporeal membrane oxygenation in children with COVID-19 and PIMS-TS during the second and third wave. Lancet Child Adolesc Health. 2022;6(4):e14–e5. https://doi.org/10.1016/S2352-4642(22)00065-7.
Registry Dashboard of ECMO-Supported COVID-19 Patient Data. https://elso.org/registry/fullcovid-19registrydashboard.aspx (2023).
Dyamenahalli U, Tuzcu V, Fontenot E, Papagiannis J, Jaquiss RD, Bhutta A, et al. Extracorporeal membrane oxygenation support for intractable primary arrhythmias and complete congenital heart block in newborns and infants: short-term and medium-term outcomes. Pediatr Crit Care Med. 2012;13(1):47–52. https://doi.org/10.1097/PCC.0b013e3182196cb1.
Ghaleb S, Thiagarajan RR, Cooper DS, Czosek RJ. Outcomes of pediatric patients treated with extracorporeal membrane oxygenation for intractable supraventricular arrhythmias. Pediatr Crit Care Med. 2020;21(8):e547–e56. https://doi.org/10.1097/PCC.0000000000002315.
Nasr VG, Faraoni D, DiNardo JA, Thiagarajan RR. Adverse outcomes in neonates and children with pulmonary artery hypertension supported with ECMO. ASAIO J. 2016;62(6):728–31. https://doi.org/10.1097/MAT.0000000000000419.
Kaestner M, Schranz D, Warnecke G, Apitz C, Hansmann G, Miera O. Pulmonary hypertension in the intensive care unit. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart. 2016;102(Suppl 2):ii57–66. https://doi.org/10.1136/heartjnl-2015-307774.
Dhillon R, Pearson GA, Firmin RK, Chan KC, Leanage R. Extracorporeal membrane oxygenation and the treatment of critical pulmonary hypertension in congenital heart disease. Eur J Cardiothorac Surg. 1995;9(10):553–6. https://doi.org/10.1016/s1010-7940(05)80004-1.
Chiel LE, Winthrop ZA, Fynn-Thompson F, Midyat L. Extracorporeal membrane oxygenation and paracorporeal lung assist devices as a bridge to pediatric lung transplantation. Pediatr Transplant. 2022;26(5):e14289. https://doi.org/10.1111/petr.14289.
Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145(3):862–7; discussion 7-8. https://doi.org/10.1016/j.jtcvs.2012.12.022.
Rehder KJ, Turner DA, Hartwig MG, Williford WL, Bonadonna D, Walczak RJ, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care. 2013;58(8):1291–8. https://doi.org/10.4187/respcare.02155.
Thompson K, Staffa SJ, Nasr VG, Zalieckas JM, Fynn-Thompson F, Boyer D, et al. Mortality after lung transplantation for children bridged with extracorporeal membrane oxygenation. Ann Am Thorac Soc. 2022;19(3):415–23. https://doi.org/10.1513/AnnalsATS.202103-250OC. This study used United Network for Organ Sharing data to analyze lung transplant recipients ≤20 years from January 2004 to August 2019, with the goal of comparing outcomes in children bridged with ECMO, supported with mechnical ventilation, and given neither support. While ECMO to bridge children receiving lung transplanation has increased, multivariable analysis showed that bridging with both ECMO and mechanical ventilation was associated with increased in-hospital mortality after lung transplantation. This association was not present for a composite outcome of mortality and retransplantation at 1 year
Toprak D, Midyat L, Freiberger D, Boyer D, Fynn-Thompson F, Visner G. Outcomes of mechanical support in a pediatric lung transplant center. Pediatr Pulmonol. 2017;52(3):360–6. https://doi.org/10.1002/ppul.23535.
Gazit AZ, Sweet SC, Grady RM, Huddleston CB. First experience with a paracorporeal artificial lung in a small child with pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(6):e48–50. https://doi.org/10.1016/j.jtcvs.2011.02.005.
Gazit AZ, Sweet SC, Grady RM, Boston US, Huddleston CB, Hoganson DM, et al. Recommendations for utilization of the paracorporeal lung assist device in neonates and young children with pulmonary hypertension. Pediatr Transplant. 2016;20(2):256–70. https://doi.org/10.1111/petr.12673.
Cullivan S, Murphy CA, Weiss L, Comer SP, Kevane B, McCullagh B, et al. Platelets, extracellular vesicles and coagulation in pulmonary arterial hypertension. Pulm Circ. 2021;11(3) https://doi.org/10.1177/20458940211021036.
Handler SS, Jin J, Ogawa MT, Feinstein JA, Lo C. Abnormal platelet aggregation in pediatric pulmonary hypertension. Pulm Circ. 2022;12(3):e12104. https://doi.org/10.1002/pul2.12104.
Vrigkou E, Tsangaris I, Bonovas S, Kopterides P, Kyriakou E, Konstantonis D, et al. Platelet and coagulation disorders in newly diagnosed patients with pulmonary arterial hypertension. Platelets. 2019;30(5):646–51. https://doi.org/10.1080/09537104.2018.1499890.
Bacha NC, Levy M, Guerin CL, Le Bonniec B, Harroche A, Szezepanski I, et al. Treprostinil treatment decreases circulating platelet microvesicles and their procoagulant activity in pediatric pulmonary hypertension. Pediatr Pulmonol. 2019;54(1):66–72. https://doi.org/10.1002/ppul.24190.
Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol. 2001;37(4):413–21. https://doi.org/10.1097/00005344-200104000-00008.
Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest. 2009;135(1):130–6. https://doi.org/10.1378/chest.08-1323.
Gudmundsdóttir IJ, McRobbie SJ, Robinson SD, Newby DE, Megson IL. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem Biophys Res Commun. 2005;337(1):382–5. https://doi.org/10.1016/j.bbrc.2005.09.060.
Conrad SA, Broman LM, Taccone FS, Lorusso R, Malfertheiner MV, Pappalardo F, et al. The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in Extracorporeal Life Support. A position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. 2018;198(4):447–51. https://doi.org/10.1164/rccm.201710-2130CP.
Sperotto F, Daverio M, Amigoni A, Gregori D, Dorste A, Allan C, et al. Trends in in-hospital cardiac arrest and mortality among children with cardiac disease in the intensive care unit: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(2):e2256178. https://doi.org/10.1001/jamanetworkopen.2022.56178.
Lasa JJ, Rogers RS, Localio R, Shults J, Raymond T, Gaies M, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge: a report from the American Heart Association's Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation. 2016;133(2):165–76. https://doi.org/10.1161/CIRCULATIONAHA.115.016082.
Nellis ME, An A, Mahmood H, Prishtina F, Hena Z, Karam O. Epidemiology of anticoagulation for children supported by extracorporeal membrane oxygenation in the United States: a Pediatric Hospital Information System database study. Perfusion. 2023; https://doi.org/10.1177/02676591221151027. Data from pediatric encounters in the Pediatric Health Information System database involving ECMO from 2012 to 2020 showed that the majority (95%) of children on ECMO in the U.S. receive unfractionated heparin anticoagulation, with a notable increase in the use of direct thrombin inhibitors over time.
Lahart M, Said AS. Extracorporeal anticoagulation with bivalirudin; is the best still to come? ASAIO J. 2022;68(11):e223. https://doi.org/10.1097/MAT.0000000000001705.
Schill MR, Douds MT, Burns EL, Lahart MA, Said AS, Abarbanell AM. Is anticoagulation with bivalirudin comparable to heparin for pediatric extracorporeal life support? Results from a high-volume center. Artif Organs. 2021;45(1):15–21. https://doi.org/10.1111/aor.13758.
Seelhammer TG, Hamzah M, Wieruszewski P. Antithrombin replacement and extracorporeal membrane oxygenation: the time is ripe for a simpler solution. ASAIO J. 2022;68(10):e166–e7. https://doi.org/10.1097/MAT.0000000000001704.
VanderPluym CJ, Cantor RS, Machado D, Boyle G, May L, Griffiths E, et al. Utilization and outcomes of children treated with direct thrombin inhibitors on paracorporeal ventricular assist device support. ASAIO J. 2020;66(8):939–45. https://doi.org/10.1097/MAT.0000000000001093.
Zamora IJ, Shekerdemian L, Fallon SC, Olutoye OO, Cass DL, Rycus PL, et al. Outcomes comparing dual-lumen to multisite venovenous ECMO in the pediatric population: the Extracorporeal Life Support Registry experience. J Pediatr Surg. 2014;49(10):1452–7. https://doi.org/10.1016/j.jpedsurg.2014.05.027.
Aydin SI, Duffy M, Rodriguez D, Rycus PT, Friedman P, Thiagarajan RR, et al. Venovenous extracorporeal membrane oxygenation for patients with single-ventricle anatomy: a registry report. J Thorac Cardiovasc Surg. 2016;151(6):1730–6. https://doi.org/10.1016/j.jtcvs.2015.12.029.
Nair AB, Oishi P. Venovenous extracorporeal life support in single-ventricle patients with acute respiratory distress syndrome. Front Pediatr. 2016;4:66. https://doi.org/10.3389/fped.2016.00066.
Boyle K, Felling R, Yiu A, Battarjee W, Schwartz JM, Salorio C, et al. Neurologic outcomes after extracorporeal membrane oxygenation: a systematic review. Pediatr Crit Care Med. 2018;19(8):760–6. https://doi.org/10.1097/PCC.0000000000001612.
Ijsselstijn H, Schiller RM, Holder C, Shappley RKH, Wray J, Hoskote A. Extracorporeal Life Support Organization (ELSO) guidelines for follow-up after neonatal and pediatric extracorporeal membrane oxygenation. ASAIO J. 2021;67(9):955–63. https://doi.org/10.1097/MAT.0000000000001525.
Sadhwani A, Cheng H, Stopp C, Rollins CK, Jolley MA, Dunbar-Masterson C, et al. Early neurodevelopmental outcomes in children supported with ECMO for cardiac indications. Pediatr Cardiol. 2019;40(5):1072–83. https://doi.org/10.1007/s00246-019-02115-1.
Funding
M.M.B’s institution received funding from the National Institute of Neurological Disorders and Stroke (R01NS106292).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nadkarni, A.S., Delany, D.R., Schramm, J. et al. ECMO Considerations in the Pediatric Cardiac Population. Curr Pediatr Rep 11, 86–95 (2023). https://doi.org/10.1007/s40124-023-00292-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-023-00292-5