Abstract
Introduction
Recently, there has been a progressive shift from simple water-adding medications towards complex multi-action combined formulas aimed at disrupting different mechanisms within the dry eye disease (DED) vicious cycle. This study evaluated the efficacy and tolerability of Trimix eye drops (Off Health Italia, Italy), a combination of viscosity-enhancing hyaluronic acid, trehalose, and cationic liposomes comprising stearylamine and phospholipids, in patients with DED.
Methods
In this prospective, pilot study patients diagnosed with mild to moderate DED were enrolled and treated with Trimix eye drops three times daily for 2 months. Ocular surface workup was performed before (V0) and after therapy (V1) by means of IDRA (SBM Sistemi, Turin, Italy), for the measurement of (i) noninvasive break-up time (NIBUT); (ii) tear meniscus height (TMH); (iii) lipid layer thickness (LLT); (iv) infrared meibography (percentage of meibomian gland loss); (v) bulbar redness (Efron scale). Treatment tolerability was scored on a visual analog scale ranging from 0 (none/not at all) to 100 (much/very) for eight questions. Ocular discomfort symptoms were scored using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire.
Results
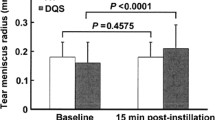
Overall, 25 subjects (mean age 60.32 ± 14.55 years) were included in the study. At V1, TMH, NIBUT, and LLT significantly increased compared to V0 (from 0.29 ± 0.06 to 0.46 ± 0.06 mm, 6.34 ± 2.61 to 7.58 ± 2.52 s, and from 63.26 ± 17.15 to 68.42 ± 15.63 nm, respectively; all P < 0.04). Concerning ocular discomfort symptoms, SPEED score significantly improved at V1 (from 16.63 ± 6.32 to 8.30 ± 5.98; P < 0.001); moreover, treatment tolerability was high for all eight items investigated.
Conclusions
Two-month treatment with Trimix formulation improved objective signs and subjective symptoms in patients with DED, showing also a good tolerability profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dry eye disease (DED) represents one the most frequent eye disorders encountered in clinical practice, with a reported prevalence ranging between 5% and 50% in the adult population. |
Tear supplementation represents the mainstay of current DED management; in the last few years, there has been a progressive shift towards complex multi-action combined formulas targeting and disrupting different key mechanisms within the DED vicious cycle. |
Trimix eye drop is a combination of viscosity-enhancing hyaluronic acid, trehalose, and cationic liposomes comprising stearylamine and phospholipids, which has shown promising results in both animal and ex vivo models of DED. |
Trimix eye drops administered three times daily for 2 months led to a significant improvement in noninvasive breakup time (NIBUT), tear meniscus height (TMH), and lipid layer thickness (LLT) in patients with mild to moderate DED. |
Trimix eye drops significantly also improved ocular discomfort symptoms while showing a good tolerability profile. |
Introduction
Dry eye disease (DED) is among the most common conditions affecting the eye, with a prevalence ranging between 5% and 50% in the adult population [1]. By affecting both daily quality of life and visual function, DED has become a growing public health concern with significant socio-economic impact. Loss of homeostasis of the ocular surface and the subsequent tear film instability, hyperosmolarity, and inflammation characterize the vicious circle of the disease [2]. Tear film instability, which acts as the hallmark of DED, is caused by changes in lipid layer function that affect quantity, quality, and availability of the tear fluid. Subsequent anatomical and functional epithelial changes stimulate both subclinical and clinical apparent inflammation. Epithelial malfunction caused by additional factors such as friction, adverse environmental factors, ocular surface irritation, and nerve impairment, can lead to further injury, increasing the inflammatory cascade and hence contributing to the maintenance of the aforementioned tear film instability. Furthermore, the impairment of corneal nerve plexa will affect both epithelial physiological turnover and tear film production [3, 4].
Given the complex and multifactorial nature of DED, it becomes of crucial importance to adopt a proper diagnostic approach, aimed at determining the relevance of each factor, so that an effective, long-lasting, and customized treatment may be administered and modified when necessary [5, 6]. Among the different available therapeutic strategies, tear supplementation represents the mainstay of current DED management. This approach consists of the administration of tear substitutes specifically designed to restore and maintain a structurally and functionally healthy ocular surface [7]. In the last few years, thanks to growing understanding of the pathophysiology, there has been a progressive shift in DED treatment, from simple water-adding medications towards complex multi-action combined formulas targeting and disrupting different key mechanisms within the DED vicious cycle [8, 9].
Trimix formulation (Off Health Italia, Italy) is the combination of viscosity-enhancing hyaluronic acid (HA), trehalose, and cationic liposomes comprising stearylamine and phospholipids. To date, the efficacy of Trimix eye drop in counteracting the vicious circle underlying DED has been demonstrated in a preclinical setting where it showed a significant protective effect on experimentally damaged rabbit corneal epithelial cells when compared to linear HA and trehalose alone; furthermore, its antioxidant action has been evaluated in vitro on a sample of irradiated human corneal cells, where it significantly reduced the level of reactive oxygen species [10].
The study aimed to evaluate the effects of Trimix eye drop on objective ocular surface parameters and subjective symptoms of ocular discomfort in patients with DED, and to further assess treatment the tolerability profile.
Methods
This prospective, single-center pilot study enrolled adult patients diagnosed with mild to moderate DED (levels 1 and 2 according to Dry Eye Workshop [DEWS] severity grading scheme) [11]. The study was approved by the local ethics committee (Comitato Etico Regione Calabria—Sezione Area Centro, protocol n. 218/2021) and conducted in accordance with Good Clinical Practice and the ethical principles laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained from each patient before any procedure. Consecutive patients over 18 years of age with a confirmed diagnosis of DED who attended the ocular surface office for routine control visits reporting a poor control of ocular discomfort symptoms with tear substitutes were screened for enrolment. The diagnosis of DED was reached according to DEWS II criteria: ocular surface disease index (OSDI) score at least 13 plus noninvasive tear breakup time (NIBUT) less than 10 s [2]. Contact lens wearing, previous corneal surgery, active ocular diseases including allergy, uncontrolled systemic diseases, and use of concomitant topical medications other than tear substitutes (e.g., corticosteroids, cyclosporine, antibiotics) in the last month were considered exclusion criteria. All patients that fulfilled study criteria were dispensed HA 0.2%-based eye drop (Icross, Off Health Italia, Italy) three times daily for 7 days (washout period). Ocular surface workup was performed before (V0) and after 2 months of therapy (V1) with Trimix eye drop instilled three times daily using IDRA (SBM Sistemi, Turin, Italy), an all-in-one device which allows the automated measurement of the following examinations: (i) NIBUT; (ii) tear meniscus height (TMH); (iii) lipid layer thickness (LLT); (iv) infrared meibography; (v) bulbar redness [12]. NIBUT was measured without the need for fluorescein dye after asking the patient to blink three consecutive times and then hold the eyes open. The measurement was repeated three times, and the mean value was recorded. TMH was measured in millimeters along the lower lid margin immediately below the pupil. LLT was estimated by observing the interference pattern and colors of the moving lipid tear film. Infrared meibography was performed in the lower eyelid, and meibomian gland loss (MGL) was calculated by means of ImageJ software as the percentage of gland loss in relation to the total tarsal area of the lid [13]. Concerning bulbar redness, an image of the conjunctiva was acquired and then compared with Efron scale for reaching a score.
Treatment tolerability was scored on a visual analog scale ranging from 0 (none/not at all) to 100 (much/very), as previously described [14]. Briefly, the questionnaire contained four positive questions covering ocular comfort, soothing, moistening/lubricating, and visual clarity (How comfortable is the drop in your eye? How soothing is the drop in your eye? How moistening/lubricating is the drop in your eye? How clear is your vision with the drop in your eye?) and four negative questions covering sensations of ocular stickiness, blurring, burning/stinging, and discomfort (How much stickiness do you have with the drop in your eye? How much blur do you have with the drop in your eye? How much burning/stinging do you have with the drop in your eye? How much discomfort do you have with the drop in your eye?). Ocular discomfort symptoms were scored at V0 and V1 using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire.
Power Analysis
To determine the sample size, a power analysis based on the results of a previous study evaluating another tear substitute was conducted [15]. Based on this analysis, a samples size of 19 patients would be required to detect a difference of 1.2 ± 1.7 s in NIBUT with a power of 0.80 and a P value of 0.05. However, to ensure adequate reliability, we aimed for a larger sample size of 25 patients.
Statistical Analysis
Statistical analysis was conducted using R (version 4.0.0) and RStudio (version 1.2.5042) software. The Kolmogorov–Smirnov test was used to assess the normality of data. As a result of the non-normal distribution, the Wilcoxon test was used to compare ocular surface parameters before and after treatment. A P value less than 0.05 was considered statistically significant.
Results
Overall, 25 patients with DED whose ocular discomfort symptoms were poorly controlled using different types of tear substitutes containing HA (18/25), carboxymethylcellulose (4/25), or hydroxypropylmethylcellulose (3/25) were included in the study. Of these, 10 patients were male, 15 female, and the mean age was 60.32 years (± 14.55). All patients regularly instilled the eye drops for the entire duration of the study. Changes of ocular surface parameters from V0 to V1 after the use of Trimix eye drops are summarized in Table 1.
In particular, TMH, NIBUT, and LLT significantly increased after treatment (from 0.29 ± 0.06 to 0.46 ± 0.06 mm, 6.34 ± 2.61 to 7.58 ± 2.52 s, and from 63.26 ± 17.15 to 68.42 ± 15.63 nm, respectively; all P < 0.04). Conversely, bulbar redness score and MGL showed no significant changes over time (both P > 0.12).
With regards to ocular discomfort symptoms, SPEED score significantly improved after treatment (from 16.63 ± 6.32 to 8.30 ± 5.98; P < 0.001). Moreover, the treatment was well tolerated, as demonstrated by the mean scores on all eight items in the tolerability questionnaire (Fig. 1).
Discussion
DED pathogenesis is characterized by a complex loop of cyclic events connecting tear film instability and hyperosmolarity, inflammatory response, and metaplastic changes of ocular surface epithelia. Any therapeutic approach must be targeted to breakdown the loop with the aim at preventing disease persistence and progression. Data from the present study demonstrated an amelioration of ocular discomfort symptoms and an improvement of the main objective parameters assessing the status of the ocular surface (namely TMH, NIBUT, and LLT) in a cohort of patients with DED treated with a new commercially available multi-action tear substitute formula comprising cross-linked HA, trehalose, and liposomes. In particular, the beneficial effects of the Trimix formulation were documented by resultant enhanced and much more stable lubrication of the ocular surface as reflected by an increased TMH and NIBUT; in parallel, a significant improvement of ocular discomfort symptoms evaluated by means of the SPEED questionnaire was also recorded. Tolerability of the study eye drop was good as demonstrated by the high scores related to the positive questions and the low scores related to the negative questions.
The significant improvement of TMH, NIBUT, and LLT—markers of the hydration of the ocular surface system and stability of the tear film—may be a consequence of reduced evaporation induced by the treatment. All these effects can be related to the properties of cross-linked HA which forms a dynamic viscoelastic solution in water contributing to create a mechanical protection for cells whilst retaining water and maintaining a resistant surface lubrication [16]. The effects of HA are enhanced by the concomitant presence of trehalose and cationic liposomes. The former is effective in maintaining the integrity of the phospholipid layers, preserving labile cell proteins against dessication and protecting cells against oxidative damage. It also accelerates the corneal healing process, reduces the level of inflammatory cytokines, and helps restore an osmotic balance of the ocular surface. The latter addresses LLT which is known to play an important role in tear film stabilization. Not by chance, up to 80% of patients with DED suffer from meibomian gland dysfunction (MGD) which then causes quantitative and qualitative alterations of lipid layer [17]. Indeed, it has been demonstrated that a thicker lipid layer results in a more stable tear film with longer NIBUT, whereas thinner and more heterogeneous values are associated with a less stable tear film. Cationic liposomes of the Trimix formula functionally mimic a healthy tear film. They provide nonpolar and polar lipids to replenish the lipid layer and increase its thickness, while simultaneously improving the stability of its interface with the aqueous phase of the tear film. In addition, the positive charge provided by cationic liposomes contributes to the generation of electrostatic forces that help in the adsorption of tear film-soluble proteins at the lipid layer interface and further stabilize this layer. The positive charge of the oil nanodroplets also helps in the homogeneous spreading of the tear substitute on the negatively charged ocular surface. The nonionic water-soluble surfactants aid in the stabilization of this interface. The hypo-/iso-osmolarity of the solution also contributes toward the modulation of the hyperosmolarity of the tear film. Furthermore, the good performance of lipid-based products in DED management is based on their ancillary anti-inflammatory properties [18].
The present pilot study suffers from limitations that deserve mentioning. In particular, the study design did not include a control arm and the noninvasive diagnostic workup did not take into account tear osmolarity that is one of the key mechanisms of DED. Future randomized controlled clinical trials should address both these issues to provide more robust evidence about the efficacy of this novel tear substitute.
Conclusions
Two-month treatment with Trimix formulation improved objective signs and subjective symptoms in a cohort of patients with mild to moderate DED who also reported a high tolerability for the study product. The efficacy of this therapy in managing the spectrum of DED relies on its multi-active combination, whose components synergistically modulate the different etiological factors of the disease.
References
Mencucci R, Favuzza E, Decandia G, Cennamo M, Giansanti F. Hyaluronic acid/trehalose ophthalmic solution in reducing post-cataract surgery dry eye signs and symptoms: a prospective, interventional, randomized, open-label study. J Clin Med. 2021;10:4699.
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83.
Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13.
Rolando M, Refojo MF, Kenyon KR. Tear water evaporation and eye surface diseases. Ophthalmologica. 1985;190:147–9.
Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease: a P.I.C.A.S.S.O. board review. Br J Ophthalmol. 2021;105:446–53.
Di Cello L, Pellegrini M, Vagge A, et al. Advances in the noninvasive diagnosis of dry eye disease. Appl Sci. 2021;11:10384.
Fallacara A, Vertuani S, Panozzo G, Pecorelli A, Valacchi G, Manfredini S. Novel artificial tears containing cross-linked hyaluronic acid: an in vitro re-epithelialization study. Molecules. 2017;22:2104.
Caretti L, La Gloria Valerio A, Piermarocchi R, et al. Efficacy of carbomer sodium hyaluronate trehalose vs hyaluronic acid to improve tear film instability and ocular surface discomfort after cataract surgery. Clin Ophthalmol. 2019;13:1157–63.
Fariselli C, Giannaccare G, Fresina M, Versura P. Trehalose/hyaluronate eyedrop effects on ocular surface inflammatory markers and mucin expression in dry eye patients. Clin Ophthalmol. 2018;12:1293–300.
Angelinetta C, Pastoris O, Pianca U, Regola E, Vicini R. In vitro evaluation of the protective action of a product against IV rays on reconstructed human ocular epithelium. Data on file, protocol n. 2002128V2-1. Bio Basic Europe Srl. 2020.
DEWS Definition and Classification Subcommittee. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Work Shop (2007). Ocul Surf. 2007;5:75–92.
Vigo L, Pellegrini M, Bernabei F, Carones F, Scorcia V, Giannaccare G. Diagnostic performance of a novel noninvasive workup in the setting of dry eye disease. J Ophthalmol. 2020;2020:5804123.
Bernabei F, Versura P, Pellegrini M, et al. Longitudinal analysis of infrared meibography in patients undergoing hematopoietic stem cell transplantation. Cornea. 2020;39:812–7.
Simmons PA, Carlisle-Wilcox C, Chen R, Liu H, Vehige JG. Efficacy, safety, and acceptability of a lipid-based artificial tear formulation: a randomized, controlled, multicenter clinical trial. Clin Ther. 2015;37:858–68.
Robert PY, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol. 2016;26:546–55.
Nakamura M, Hikida M, Nakano T, Ito S, Hamano T, Kinoshita S. Characterization of water retentive properties of hyaluronan. Cornea. 1993;12:433–6.
Wizert A, Iskander DR, Cwiklik L. Organization of lipids in the tear film: a molecular-level view. PLoS ONE. 2014;9:92461.
Daull P, Amrane M, Ismail D, et al. Cationic emulsion-based artificial tears as a mimic of functional healthy tear film for restoration of ocular surface homeostasis in dry eye disease. J Ocul Pharmacol Ther. 2020;36:355–65.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Luca Vigo, Carlotta Senni, Marco Pellegrini, Aldo Vagge, Lorenzo Ferro Desideri, Giuseppe Giannaccare. The first draft of the manuscript was written by Carlotta Senni and Lorenzo Ferro Desideri. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Luca Vigo, Carlotta Senni, Marco Pellegrini, Aldo Vagge, Lorenzo Ferro Desideri, Francesco Carones, Vincenzo Scorcia and Giuseppe Giannaccare all confirm that they have no conflicts of interest to disclose.
Compliance with ethics guidelines
Informed consent was acquired from all the participants, and the study was carried out in accordance with the Declaration of Helsinki of 1964 and its later amendments, with approval from the local institutional ethics committee (Comitato etico Regione Calabria, protocol number 218. Date of approval 16 September 2021).
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vigo, L., Senni, C., Pellegrini, M. et al. Effects of a New Formulation of Multiple-Action Tear Substitute on Objective Ocular Surface Parameters and Ocular Discomfort Symptoms in Patients with Dry Eye Disease. Ophthalmol Ther 11, 1441–1447 (2022). https://doi.org/10.1007/s40123-022-00518-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00518-7