Abstract
Introduction
Multi-modal analgesia is desirable for the management of acute pain since it can provide effective pain relief at lower doses, thereby aiding tolerability. Co-crystal of tramadol-celecoxib (CTC) provides effective analgesia in models of acute pain. Co-crystallization can alter the pharmacokinetics of individual components, potentially improving tolerability. We sought to better understand the safety and tolerability of CTC in patients with acute postoperative pain.
Methods
We conducted a pooled analysis of safety data from three phase 3 randomized controlled trials in adults with acute moderate-to-severe pain following oral surgery, bunionectomy, and elective abdominal hysterectomy. We present data for CTC 200 mg twice daily (BID) and its comparators: tramadol 50 mg four times daily (QID) (one trial), tramadol 100 mg QID (two trials), celecoxib 100 mg BID (two trials), and placebo (three trials).
Results
In total, n = 551 patients received CTC 200 mg BID, n = 183 received tramadol 50 mg QID, n = 368 received tramadol 100 mg QID, n = 388 received celecoxib 100 mg BID, and n = 274 received placebo. The prevalence of adverse events (AEs) related to study drug up to 48 h was numerically lower with CTC 200 mg BID (35.9%) than with tramadol 50 mg QID (47.5%) and 100 mg QID (44.8%) but greater than with celecoxib 100 mg BID (12.4%) and placebo (20.4%). The most frequent AEs related to study drug up to 48 h were somnolence, nausea, dizziness, and vomiting, which occurred more frequently in patients receiving tramadol 100 mg QID than in those receiving CTC 200 mg BID.
Conclusion
CTC 200 mg BID appears to be better tolerated than tramadol 100 mg QID, possibly because of reduced total exposure to tramadol. This may contribute to a more favorable benefit-risk profile for CTC versus individual components, making it a promising treatment for acute pain.
Trial Registration
ClinicalTrials.gov identifiers: NCT03108482, NCT02982161 (EudraCT: 2016-000592-24), NCT03062644 (EudraCT: 2016-000593-38).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
If poorly managed, acute pain can impact health and quality of life and may develop into chronic pain |
There is a need for well-tolerated multi-modal analgesics that can reduce exposure to opioids for patients with acute moderate-to-severe pain |
Our analysis sought to better understand the tolerability of co-crystal of tramadol-celecoxib (CTC) 200 mg twice daily (BID) compared with tramadol 50 mg four times daily (QID) and 100 mg QID and celecoxib 100 mg BID by pooling data from three phase 3 randomized controlled trials in patients with acute moderate-to-severe postoperative pain |
What was learned from the study? |
CTC 200 mg BID reduced exposure to tramadol and appeared to be better tolerated than tramadol 100 mg QID and to have similar tolerability to tramadol 50 mg QID |
The tolerability of CTC in our pooled analysis reinforces the observations from the individual trials. Coupled with the previously reported efficacy data, our pooled analysis supports CTC as having a favorable benefit-risk profile compared with tramadol or celecoxib alone |
Introduction
Unrelieved acute pain can negatively impact health and quality of life, and it may develop into chronic pain [1, 2]. If managed insufficiently, pain can also lead to increased healthcare usage and costs [2]. Unfortunately, the management of acute pain remains inadequate for many patients [3,4,5,6]. Although many current single-agent treatments, such as opioid-based analgesics, are effective at managing acute pain, they are associated with side effects including sedation, nausea, constipation, and vomiting [7, 8]. Side effects of opioids are common in the acute-pain setting, and they vary by opioid [9, 10] and dose size [11, 12]. Indeed, risk of respiratory depression increases with increasing morphine-equivalent daily doses [13]. The avoidance of adverse events (AEs) associated with analgesic medications is an important factor for physicians prescribing treatments for acute pain, and there is an unmet need for medications with fewer side effects [7]. With opioids, there is also a potential for dependence and misuse [14,15,16], with higher opioid doses (and the speed at which plasma concentrations are achieved) influencing drug liking [17]. There is a desire for new, effective pain medications that can reduce opioid exposure and help minimize side effects and the potential for dependence.
Co-crystal of tramadol-celecoxib (CTC) is an analgesic produced by the co-crystallization of racemic tramadol hydrochloride (a centrally acting, atypical, synthetic opioid analgesic) and celecoxib (a cyclooxygenase-2 selective nonsteroidal anti-inflammatory drug). CTC represents a multi-modal approach that targets multiple complementary pain pathways, which may allow for the provision of effective analgesia with lower daily doses of tramadol and may thereby reduce the potential for adverse effects [18]. CTC is formulated as 100-mg immediate-release tablets containing 44 mg of racemic tramadol hydrochloride and 56 mg of celecoxib. CTC was approved in the USA in 2021 [19] and received European regulatory approval (in Spain) in 2023 [20]. The co-crystallization of tramadol and celecoxib within CTC modifies the physicochemical properties and bioavailability of both components, optimizing the pharmacokinetic profile compared with either agent alone or with both administered concomitantly [18, 21,22,23,24]. It is therefore important to understand how the efficacy and safety of CTC may differ from that of its individual components.
The efficacy and safety of CTC have been assessed in three phase 3 clinical trials in adults with acute, moderate-to-severe postoperative pain. In addition to providing a better benefit-risk balance compared with either tramadol or celecoxib alone [25,26,27,28], CTC permitted a lower cumulative daily dosing of tramadol [26] and reduced the need for opioid-containing rescue medication [25]. To further evaluate the safety and tolerability of CTC 200 mg twice daily (BID; the approved dose), we conducted a formal pooled analysis of data from placebo- and active-controlled phase 3 clinical trials that evaluated CTC 200 mg BID in patients with acute, moderate-to-severe pain.
Methods
We conducted an integrated analysis of safety data from the ESTEVE-SUSA-301, STARDOM1, and STARDOM2 trials. The designs of these trials have been described previously [25,26,27,28] and are summarized here and in Fig. 1. All trials were conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki, and all trials received appropriate local ethics committee approval. All patients provided written informed consent. The ESTEVE-SUSA-301 protocol was approved by an Institutional Review Board, Sterling IRB (Atlanta, GA: IRB ID: 5724) [25]. The STARDOM1 study protocol was approved by the local ethics committee for each country and/or study site. The principal investigator was from Spain, and the Spanish ethics committee was the Comité Ético de Investigación Clínica con Medicamentos del Hospital Universitario de la Princesa (Madrid), resolution no. 20/17 of 10 November 2016 [26]. The STARDOM2 study protocol was reviewed and approved by local ethics committees and all concerned competent authorities for each country and/or study site. The principal investigator was from Poland, and the Polish ethics committee was the Bioethics Committee at the Poznan University of Medical Sciences, resolution no. 17/02 of 5 January 2017 [27].
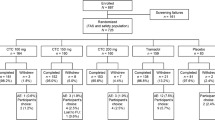
Summary of the study design and features of the ESTEVE-SUSA-301, STARDOM1, and STARDOM2 phase 3 randomized controlled trials of CTC. Adapted from Co-crystal of tramadol-celecoxib (CTC) for acute moderate-to-severe pain, Langford R et al., Current Medical Research and Opinion, copyright © 2024 The Author(s), reprinted by permission of Informa UK Limited, trading as Taylor & Francis Group, https://www.tandfonline.com. BID twice daily, CTC co-crystal of tramadol-celecoxib, QID four times daily
ESTEVE-SUSA-301
ESTEVE-SUSA-301 was a phase 3, randomized, double-blind, active- and placebo-controlled trial (NCT03108482) [25] conducted at centers in the USA. ESTEVE-SUSA-301 enrolled adult patients with moderate-to-severe pain (assessed by a pain intensity rating score of 5–9 on a numerical rating scale of 0–10) within 8 h of popliteal sciatic nerve block cessation following primary unilateral first metatarsal osteotomy, with internal fixation and no additional collateral procedure. After a screening period of ≤ 28 days, patients were admitted to the study center on the day of surgery and remained there for 3 nights. A total of 637 patients were randomized 2:2:2:1 to receive treatment with oral CTC 200 mg BID (n = 184), tramadol 50 mg four times daily (QID) (n = 183), celecoxib 100 mg BID (n = 181), or placebo QID (n = 89). Patients received eight doses of study medication during the 48-h treatment period and then returned for a follow-up visit between 5 and 9 days post-surgery. Rescue medication for pain could be administered after study medication was initiated. The first-line rescue medication was acetaminophen 1 g administered intravenously every 4–6 h, as needed (up to 4 g in 24 h). If additional rescue medication was required, patients could receive oxycodone 5 mg immediate-release tablets administered every 4–6 h, as needed (up to 30 mg in 24 h).
STARDOM1
STARDOM1 was a phase 3, randomized, double-blind, active- and placebo-controlled trial (NCT02982161; EudraCT: 2016-000592-24) [26] conducted at sites in Canada and Europe (Germany, Hungary, Italy, Poland, and Spain). STARDOM1 enrolled adult patients who had undergone an elective oral surgical procedure to extract two or more impacted third molars (including ≥ 1 mandibular molar), required bone removal, and were experiencing acute moderate-to-severe pain [defined as a rating of ≥ 45 mm on a 100-mm pain intensity visual analog scale (PI-VAS), measured within 6 h of the procedure] as a result of the surgery. Patients were included if the extractions were completed without immediate complication within 28 days of screening. In STARDOM1, a total of 726 patients were randomized 2:2:2:2:1 to receive oral CTC 100 mg BID (n = 164), CTC 150 mg BID (n = 160), CTC 200 mg BID (n = 160), tramadol 100 mg QID (n = 159), or placebo QID (n = 83). Patients received 13 doses of study medication during the 72-h treatment period. Oral acetaminophen QID, up to a maximum of 4000 mg daily, was permitted as rescue medication.
STARDOM2
STARDOM2 was a phase 3, randomized, double-blind, active- and placebo-controlled trial (NCT03062644; EudraCT: 2016-000593-38) conducted at sites in Belarus, Bulgaria, Hungary, Latvia, Poland, Russia, and Spain [27]. STARDOM2 enrolled adult patients who had undergone an elective total or subtotal abdominal hysterectomy for benign conditions, completed under general anesthesia, and without immediate complications, within 28 days of screening. Patients were included if they were experiencing acute moderate-to-severe pain (defined as a rating of ≥ 45 mm on a 100-mm PI-VAS, measured within 30 h of the procedure and after cessation of postoperative anesthesia) as a result of the surgery. In STARDOM2, 1138 patients were randomized 2:2:2:2:2:1 to receive oral CTC 100 mg BID (n = 207), CTC 150 mg BID (n = 207), CTC 200 mg BID (n = 208), tramadol 100 mg QID (n = 208), celecoxib 100 mg BID (n = 206), or placebo QID (n = 102). Patients received up to 21 doses of study medication during the 120-h treatment period, after which they were followed-up for ≥ 7 days. Oral acetaminophen QID, up to a maximum of 4000 mg daily, was permitted as rescue medication.
Pooled Analysis
Our analysis pooled safety data from ESTEVE-SUSA-301, STARDOM1, and STARDOM2. Data for CTC 200 mg BID and placebo were pooled from all three trials, data for tramadol 100 mg QID were pooled from two trials (STARDOM1 and STARDOM2), data for celecoxib 100 mg BID were pooled from two trials (ESTEVE-SUSA-301 and STARDOM2), and data for tramadol 50 mg QID were available from only one study (ESTEVE-SUSA-301) (Fig. 1).
Safety Outcomes
AEs from each trial were converted to Medical Dictionary for Regulatory Affairs (MedDRA) version 21.0 definitions to ensure consistency of System Organ Classes and preferred terms across all trials. Safety outcome data included the proportions of patients who experienced treatment-emergent AEs, serious AEs, severe AEs, and AEs leading to study discontinuation, including those related to the study drug as deemed by the trial investigators. A serious AE was defined as any AE that resulted in death, was life-threatening, required inpatient hospitalization or prolongment of existing hospitalization, resulted in persistent or significant disability/incapacity, was a congenital anomaly/birth defect, or was a medically important event or reaction. Intensity/severity of AEs was defined based on the extent to which an AE affected the patient’s daily activities, with severe AEs defined as those that prevented or severely limited usual activities.
Statistical Analyses
Safety outcome data were reported using descriptive statistics. Statistical analyses and data processing were pre-defined in a Statistical Analysis Plan and performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics
For the pooled analysis, n = 551 patients received CTC 200 mg BID, n = 183 received tramadol 50 mg QID, n = 368 received tramadol 100 mg QID, n = 388 received celecoxib 100 mg BID, and n = 274 received placebo. Most patients included in the analysis were female (range for treatment groups: 82.0–94.6%), White (68.9–98.4%), and < 65 years of age (86.9–96.7%) (Table 1). There were no relevant differences between CTC 200 mg BID, tramadol 100 mg QID, celecoxib 100 mg BID, and placebo in terms of distribution by key baseline and demographic characteristics (Table 1). The tramadol 50 mg QID data were obtained from only one study and therefore differed from the pooled population in terms of having a greater proportion of black/African American patients (21.3%) than other treatment groups (0.5–8.0%). Moreover, the mean (standard deviation) exposure to study drug was also shorter in the tramadol 50 mg QID (41.4 [4.4] h) compared with the other treatment groups (which ranged from 75.7 [36.1] to 90.6 [35.3] h).
Safety
The prevalence of AEs related to study drug up to 48 h was numerically lower with CTC 200 mg BID (35.9%) than with tramadol 50 mg QID (47.5%) and 100 mg QID (44.8%) but greater than that observed with celecoxib 100 mg BID (12.4%) and placebo (20.4%) (Fig. 2 and Table S1). The study drug-related AE incidence rate up to 48 h (i.e., number of AEs related to study drug per 100 patient-hours) for CTC 200 mg BID was 2.8, which was half the rate observed for tramadol 100 mg QID (5.2) and similar to that observed with tramadol 50 mg QID (2.7). Both celecoxib 100 mg BID and placebo had lower incidence rates than those seen with CTC 200 mg BID, with incidence rates of 0.6 and 1.4, respectively.
When AEs related to study drug were assessed up to last follow-up, the prevalences were greater than when assessed up to 48 h, but the pattern across treatments remained consistent with that seen up to 48 h (Table S1). The overall AE rates at 48 h and up to last follow-up also followed a similar pattern, with a lower prevalence observed with CTC 200 mg BID versus tramadol 50 mg QID and 100 mg QID but a greater prevalence observed versus placebo and celecoxib 100 mg BID (Table S1).
The most frequent AEs related to study drug up to 48 h were somnolence, nausea, dizziness, and vomiting (Fig. 3 and Table S2). These AEs were also the most frequently observed AEs overall (Table S3). The prevalence of AEs related to study drug was greater in the tramadol 100 mg QID group than in the CTC 200 mg BID, celecoxib 100 mg BID, and placebo groups for the most frequent AEs by preferred term (Fig. 3 and Table S2).
Up to 48 h after receiving treatment, there was only one serious AE (vomiting) related to the study drug, and this occurred in the tramadol 100 mg QID group (Fig. 2 and Table S1). Severe AEs related to study drug were more frequent with tramadol 100 mg QID (14.9%) than with CTC 200 mg BID (4.9%), tramadol 50 mg QID (2.2%), celecoxib 100 mg BID (0.3%), and placebo (2.9%) (Fig. 2 and Table S1). The most frequent severe AEs related to study drug were somnolence, nausea, fatigue, vomiting, and dizziness, which occurred at a greater frequency in the tramadol 100 mg group versus the other treatment groups in the first 48 h (Fig. 4 and Table S4).
AEs leading to study discontinuation occurred in 4.6% of patients receiving tramadol 100 mg QID, 1.6% receiving tramadol 50 mg QID, 1.5% receiving CTC 200 mg BID, 1.0% receiving celecoxib 100 mg BID, and no patients receiving placebo (Fig. 2 and Table S1). The most common AEs leading to study discontinuation were nausea and vomiting, which occurred more frequently in the tramadol 100 mg QID group versus the other treatment groups. There were no deaths in any of the treatment groups (Table 2).
Discussion
This pooled analysis of three phase 3 randomized controlled trials assessed the safety and tolerability of CTC 200 mg BID versus tramadol 50 mg QID and 100 mg QID, celecoxib 100 mg BID, and placebo in patients with acute moderate-to-severe pain. The results demonstrated that the incidence of AEs related to study drug within the first 48 h of treatment was numerically lower with CTC 200 mg BID compared with tramadol 50 mg QID and 100 mg QID but greater than the incidence observed with celecoxib 100 mg BID or placebo. The safety profiles observed in our analysis are consistent with those reported for the study treatments in the individual ESTEVE-SUSA-301, STARDOM1, and STARDOM2 trials (Tables S5, S6, and S7; Fig. S1) [25,26,27] and also with those reported from the early phase trials with CTC [21, 22, 24].
Direct comparison with AE rates for tramadol in other studies is complicated for a number of reasons, namely, differences in patient populations, doses, formulations, treatment durations, and trial durations; the evaluation of patient-reported data using questionnaires that can lead to data inaccuracies inherent with this form of data collection; previous use of patient-controlled analgesia, which has been shown to increase tolerance to some analgesic-related AEs [29, 30]; and differences in allowed use of concomitant rescue medications [31]. Opioids are often associated with side effects such as somnolence, nausea, vomiting, and constipation [8,9,10, 16]. In keeping with these observations, we noted that the incidence of somnolence, nausea, vomiting, and constipation related to study drug was greater in patients receiving tramadol 100 mg QID than in those receiving CTC 200 mg BID, celecoxib 100 mg BID, or placebo. The incidence of somnolence, dizziness, and vomiting related to study drug was lower with tramadol 50 mg QID than with tramadol 100 mg QID, suggesting that these events were dose dependent. As may be expected, the incidence of these AEs was also numerically higher with the CTC 200 mg BID treatment group compared with the celecoxib 100 mg BID or placebo treatment groups, which would suggest that the safety profile of CTC is driven primarily by the effects of tramadol. Indeed, the safety profile of CTC was consistent with the expected safety profiles of the individual tramadol and celecoxib components, based on their labels [19, 32, 33], and CTC did not appear to have an additive effect. The US prescribing information for CTC [19] does contain a boxed warning reflective of its components. Due to the tramadol component of CTC, the US prescribing information for CTC contains warnings about the potential for addiction, abuse, and misuse, as well as the potential harm from respiratory depression and accidental ingestion, and the risks of concomitant use with benzodiazepines or other central nervous system depressants, interactions with drugs affecting cytochrome P450, neonatal opioid withdrawal syndrome, and the ultra-rapid metabolism of tramadol and other risk factors for life-threatening respiratory depression in children. The label highlights the need for an opioid analgesic Risk Evaluation and Management Strategy to ensure that the benefits of CTC treatment will outweigh the risks of addiction, abuse, and misuse. The risks of gastrointestinal bleeding and cardiovascular thrombotic events associated with nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors are also highlighted in the boxed warning [19].
Multi-modal analgesia is now a widely accepted approach for the management of acute pain and the concurrent limiting of opioid consumption and opioid-related AEs [2]. CTC provides a multi-modal approach to analgesia, targeting both central and peripheral pain pathways [18]. Indeed, CTC 200 mg BID has been reported to provide more effective analgesia than both tramadol 50 mg QID [25] and 100 mg QID [26] and celecoxib 100 mg BID [25] in patients with acute pain while also reducing the need for rescue medication [25,26,27]. Since rescue medications may also cause AEs, a reduction in rescue medication with CTC could help contribute to its overall tolerability profile. In addition, there may be other ways in which the tolerability of CTC may be improved compared with tramadol. For example, the multi-modal approach and the pharmacokinetics of the components in CTC allow patients to achieve adequate analgesia while also receiving lower equivalent doses of tramadol (176 mg daily) than patients receiving tramadol alone (200 or 400 mg daily). In addition to providing multi-modal analgesia, the co-crystallization of tramadol and celecoxib in CTC optimizes the bioavailability and pharmacokinetics of the individual components [21, 22, 24]. Results from pharmacokinetic studies have demonstrated that the maximum plasma concentrations of tramadol and celecoxib achieved with CTC 200 mg are lower than those observed with tramadol 100 mg and celecoxib 100 mg when administered alone [21, 22, 24]. Furthermore, the time to achieve maximum plasma concentration of tramadol with CTC is delayed compared with tramadol alone [21, 22, 24]. Slower administration of tramadol has been suggested to help improve tolerability [21, 22, 24]; the lower maximum plasma concentration of tramadol with CTC and the delay to achieve this maximum concentration may both therefore help contribute to the improved tolerability profile of CTC versus tramadol alone. This may also contribute to the observation of fewer AEs with CTC versus tramadol 100 mg QID. Indeed, we observed that the study drug-related AE rate with CTC was comparable to that seen with tramadol 50 mg QID (200 mg daily) and approximately half that seen with tramadol 100 mg QID (400 mg daily). Thus, the clinical efficacy of CTC is not accompanied by an increase in the number or severity of AEs compared with tramadol alone. CTC appears to possess a benefit-risk profile more favorable than that of either tramadol or celecoxib alone, providing more effective pain management than celecoxib and tramadol 50 mg QID [25] or better tolerability than tramadol 100 mg QID.
CTC may represent a valuable addition to the options available to physicians treating patients with acute pain, particularly after surgery. When treating postoperative pain, physicians may face numerous challenges, such as encouraging mobility during postoperative pain, discharge delays due to uncontrolled pain, and hospital readmissions due to pain [7]. CTC is administered as a regular dose, which may offer advantages versus regimens that are administered as needed and therefore require more consistent medical input. The positive benefit-risk profile and regular dosing schedule with CTC may help to reduce the occurrence of analgesia gaps and the need for rescue medication. This, in turn, may help patients achieve a quicker discharge without the need for direct nursing or other medical input to manage as-needed treatments. However, further studies may be required to establish whether these benefits of CTC will translate into reduced readmissions for pain.
Our pooled analysis has strengths and limitations that may need to be considered when interpreting the findings. Data were pooled from several trials; this increased number of patients may help to strengthen conclusions regarding the safety and tolerability of treatments. The trials employed different models of acute postoperative pain; these models were considered standard, validated, and sensitive for assessing the treatment of acute pain [34, 35]. Moreover, the models were complementary, because they comprised a complex pathophysiology involving tissue damage and inflammation resulting from surgery. Another consideration for our pooled analysis is that the treatment duration differed for each of the trials, although the overall safety follow-up time was similar (~ 7 days). Our analysis focused on safety data for up to 48 h after randomization, as most AEs were expected to appear in the first few hours of treatment and the AE profiles at follow-up were expected to have only minimal differences from those at 48 h. We also assessed safety and tolerability data up to the last follow-up, and both the pattern of adverse effects and the overall conclusions were comparable to the data after 48 h.
No statistical analyses were performed between treatment groups for AEs. Indeed, AEs are reported spontaneously and can differ greatly in their nature, severity, and duration; consequently, they differ from pre-determined endpoints and represent challenges for performing statistical analyses. Moreover, no statistical analysis was conducted to determine the contribution of rescue medication to AE rates.
A greater use of rescue medication was reported for placebo versus CTC in these studies [25,26,27], and AEs from these rescue medications may contribute more to the AE rate for placebo than for other treatments. Among patients who did not receive opioid rescue medication in ESTEVE-SUSA-301 (the only trial in this pooled analysis in which patients were permitted opioid-containing rescue medication), nausea and vomiting occurred less frequently with CTC than with tramadol alone; for patients who did receive oxycodone rescue medication, the incidence of nausea was similar for CTC and tramadol [25, 36].
In the present analysis, data for tramadol 50 mg QID were derived from only one trial. Consequently, there were some differences in patient demographics at baseline and treatment exposure for tramadol 50 mg QID compared with groups where data were pooled from two or more studies. These differences were not apparent when comparing baseline demographics in just the ESTEVE-SUSA-301 trial [25]. Thus, although we report data for tramadol 50 mg QID data for completeness, the differences in baseline demographics and treatment exposure would indicate that caution should be used when comparing with data pooled across multiple studies. Indeed, this may explain why some AEs, such as somnolence and fatigue, occurred at greater rates with pooled placebo data than with tramadol 50 mg QID (Table S2), but this was not observed when comparing these treatment groups directly in the ESTEVE-SUSA-301 trial (Table S5) [25].
Care should also be taken when generalizing our analysis, as the population may not be fully representative of a broader acute pain patient population; the majority of patients in our pooled analysis were female because of the inclusion of two trials in patients undergoing a bunionectomy or an elective abdominal hysterectomy. Moreover, patients were predominantly < 65 years of age, and care should therefore be taken when generalizing safety to older patients.
Conclusion
In this pooled safety analysis of three phase 3 randomized controlled trials representing over 42,000 patient-hours of CTC exposure in the setting of acute moderate-to-severe postoperative pain, CTC 200 mg BID had a more favorable safety and tolerability profile than tramadol 100 mg QID. The tolerability benefits of CTC, coupled with its previously reported analgesic efficacy, contribute to a more favorable benefit-risk profile versus its individual components and highlight the potential of CTC for the management of acute moderate-to-severe pain.
Data Availability
ESTEVE Pharmaceuticals S.A. will consider requests for deidentified patient-level data and supporting study documents from qualified external researchers. Approval of requests will be at the discretion of ESTEVE Pharmaceuticals S.A. and will depend on the scientific merit of the proposed research and intended use of the data. If approval is granted, a Data Sharing Agreement must be signed and access to data will be provided only if ESTEVE Pharmaceuticals S.A. has legal authority to provide the data and there are no contradictory requirements relating to regulatory filings or reviews. Proposals should be sent to esteve@esteve.com.
References
Meissner W, Coluzzi F, Fletcher D, et al. Improving the management of post-operative acute pain: priorities for change. Curr Med Res Opin. 2015;31(11):2131–43.
Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98.
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–40.
Todd KH, Ducharme J, Choiniere M, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007;8(6):460–6.
Mura P, Serra E, Marinangeli F, et al. Prospective study on prevalence, intensity, type, and therapy of acute pain in a second-level urban emergency department. J Pain Res. 2017;10:2781–8.
Butti L, Bierti O, Lanfrit R, et al. Evaluation of the effectiveness and efficiency of the triage emergency department nursing protocol for the management of pain. J Pain Res. 2017;10:2479–88.
Gan TJ, Epstein RS, Leone-Perkins ML, Salimi T, Iqbal SU, Whang PG. Practice patterns and treatment challenges in acute postoperative pain management: a survey of practicing physicians. Pain Ther. 2018;7(2):205–16.
European Society for Emergency Medicine (EUSEM). Guidelines for the management of acute pain in emergency situations. 2020. https://eusem.org/images/EUSEM_EPI_GUIDELINES_MARCH_2020.pdf. Accessed May 5, 2024.
Stegmann JU, Weber H, Steup A, Okamoto A, Upmalis D, Daniels S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr Med Res Opin. 2008;24(11):3185–96.
Singla NK, Pollak R, Gottlieb I, et al. Efficacy and safety of intravenously administered tramadol in patients with moderate to severe pain following bunionectomy: a randomized, double-blind, placebo-controlled, dose-finding study. Pain Ther. 2020;9(2):545–62.
Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 Suppl):6S-11S.
Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–6.
Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8(12):e024086.
Dunn KE, Bergeria CL, Huhn AS, Strain EC. A systematic review of laboratory evidence for the abuse potential of tramadol in humans. Front Psychiatry. 2019;10:704.
Reines SA, Goldmann B, Harnett M, Lu L. Misuse of tramadol in the United States: an analysis of the National Survey of Drug Use and Health 2002–2017. Subst Abuse. 2020;14:1178221820930006.
Edinoff AN, Kaplan LA, Khan S, et al. Full opioid agonists and tramadol: pharmacological and clinical considerations. Anesth Pain Med. 2021;11(4):e119156.
Marsch LA, Bickel WK, Badger GJ, et al. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299(3):1056–65.
Almansa C, Mercè R, Tesson N, Farran J, Tomàs J, Plata-Salamán CR. Co-crystal of tramadol hydrochloride–celecoxib (ctc): a novel API–API co-crystal for the treatment of pain. Cryst Growth Des. 2017;17(4):1884–92.
U.S. Food and Drug Adminstration. Highlights of prescribing information: SELGENTIS (celecoxib and tramadol hydrochloride) tablets, for oral use, C-IV. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213426s000lbl.pdf. Accessed May 5, 2024.
Centro de información online de medicamentos de la Agencia Española de Medicamentos y Productos Sanitarios (CIMA). Velyntra 44 mg/56 mg comprimidos recubiertos con película. 2023. https://cima.aemps.es/cima/publico/detalle.html?nregistro=89051. Accessed May 5, 2024.
Videla S, Lahjou M, Vaqué A, et al. Single-dose pharmacokinetics of co-crystal of tramadol-celecoxib: results of a four-way randomized open-label phase I clinical trial in healthy subjects. Br J Clin Pharmacol. 2017;83(12):2718–28.
Videla S, Lahjou M, Vaqué A, et al. Pharmacokinetics of multiple doses of co-crystal of tramadol-celecoxib: findings from a four-way randomized open-label phase I clinical trial. Br J Clin Pharmacol. 2018;84(1):64–78.
Port A, Almansa C, Enrech R, Bordas M, Plata-Salamán CR. Differential solution behavior of the new API–API co-crystal of tramadol–celecoxib (CTC) versus its constituents and their combination. Cryst Growth Des. 2019;19(6):3172–82.
Cebrecos J, Carlson JD, Encina G, et al. Celecoxib-tramadol co-crystal: a randomized 4-way crossover comparative bioavailability study. Clin Ther. 2021;43(6):1051–65.
Viscusi ER, de Leon-Casasola O, Cebrecos J, et al. Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: a phase 3, randomized, double-blind, factorial, active- and placebo-controlled trial. Pain Pract. 2023;23(1):8–22.
Langford R, Pogatzki-Zahn EM, Morte A, et al. Co-crystal of tramadol-celecoxib versus tramadol or placebo for acute moderate-to-severe pain after oral surgery: randomized, double-blind, phase 3 trial (STARDOM1). Adv Ther. 2024;41(3):1025–45.
Langford R, Morte A, Sust M, et al. Efficacy and safety of co-crystal of tramadol-celecoxib (CTC) in acute moderate-to-severe pain after abdominal hysterectomy: a randomized, double-blind, phase 3 trial (STARDOM2). Eur J Pain. 2022;26(10):2083–96.
Langford R, Margarit C, Morte A, et al. Co-crystal of tramadol-celecoxib (CTC) for acute moderate-to-severe pain. Curr Med Res Opin. 2024;40(3):455–68.
Kim S, Song IA, Lee B, Oh TK. Risk factors for discontinuation of intravenous patient-controlled analgesia after general surgery: a retrospective cohort study. Sci Rep. 2023;13(1):18318.
Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87(1):36–46.
Grovle L, Hasvik E, Haugen AJ. Rescue and concomitant analgesics in placebo-controlled trials of pharmacotherapy for neuropathic pain and low back pain. Pain. 2020;161(1):3–10.
Janssen. Highlights of prescribing information: ULTRAM® (tramadol hydrochloride) tablets, for oral use, C-IV. 2023. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/ULTRAM-pi.pdf. Accessed May 5, 2024.
Pfizer Inc. Highlights of prescribing information: CELEBREX® (celecoxib) capsules, for oral use. 2021. https://labeling.pfizer.com/showlabeling.aspx?id=793. Accessed May 5, 2024.
European Medicines Agency. Guideline on the clinical development of medicinal products intended for the treatment of pain. 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-medicinal-products-intended-treatment-pain-first-version_en.pdf. Accessed May 5, 2024.
Cooper SA, Desjardins PJ, Turk DC, et al. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain. 2016;157(2):288–301.
Viscusi ER, de Leon-Casasola O, Cebrecos J, et al. Abstract no. 435: Celecoxib-tramadol co-crystal in patients with moderate-to-severe pain following bunionectomy with osteotomy: secondary analyses of a phase 3, randomised, double-blind, factorial, active-and placebo-controlled trial (p. 175). Abstract book: 12th congress of the European Pain Federation (EFIC). 2022. https://efic-congress.org/wp-content/uploads/2022/04/ABSTRACT-BOOK-2022.pdf. Accessed May 5, 2024.
Acknowledgements
We thank all patients and investigators involved in the ESTEVE-SUSA-301, STARDOM1, and STARDOM2 trials.
Medical Writing/Editorial Assistance
Medical writing support, including developing a draft outline and subsequent drafts in consultation with the authors, collating author comments, and completing the required copyediting, fact checking, and referencing, was provided by Daniel Binks, PhD, at Aspire Scientific Limited (Bollington, UK).
Funding
This study was funded by ESTEVE Pharmaceuticals S.A., Barcelona, Spain. Medical writing support was funded by ESTEVE Pharmaceuticals S.A., Barcelona, Spain. The journal’s Rapid Service and Open Access Fees were paid for by the funder. Scientists employed by the funder participated in the study design and conduct, data review and interpretation, and drafting of the article. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
Eugene R. Viscusi, Richard Langford, Adelaida Morte, Anna Vaqué, Jesús Cebrecos, Mariano Sust, and Oscar de Leon-Casasola conceived or designed the study. Jesús Cebrecos contributed to data collection. All authors contributed to data analysis and/or interpretation; drafted, edited, and/or reviewed drafts of the manuscript; approved the final version for submission; and agree to be accountable for the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Eugene R. Viscusi reports consulting fees from ESTEVE Pharmaceuticals, Heron Therapeutics, Merck, Orion Pharma, Salix Pharmaceuticals, and Vertex Pharmaceuticals. Richard Langford reports consulting and speaker fees from Avenue Therapeutics, BioQ Pharma, Camurus, Compass, Eli Lilly, Grünenthal GmbH, Grünenthal Ltd, GSK, Heron Therapeutics, Medincell, Mundipharma, Pfizer, and Sintetica. Adelaida Morte, Anna Vaqué, Jesús Cebrecos, Mariano Sust, and José María Giménez-Arnau are employees of ESTEVE Pharmaceuticals. Oscar de Leon-Casasola reports personal fees for advisory board membership from ESTEVE Pharmaceuticals during the conduct of the study, and from ESTEVE Pharmaceuticals, Merck, and Stimgenics Medtronic outside of the submitted work.
Ethical Approval
All trials were conducted in compliance with Good Clinical Practice guidelines and the Declaration of Helsinki, and all trials received appropriate local ethics committee approval. All patients provided written informed consent. The ESTEVE-SUSA-301 protocol was approved by an Institutional Review Board, Sterling IRB (Atlanta, GA; IRB ID: 5724). The STARDOM1 study protocol was approved by the local ethics committee for each country and/or study site. The principal investigator was from Spain, and the Spanish ethics committee was the Comité Ético de Investigación Clínica con Medicamentos del Hospital Universitario de la Princesa (Madrid), resolution no. 20/17 of 10 November 2016. The STARDOM2 study protocol was reviewed and approved by local ethics committees and all concerned competent authorities for each country and/or study site. The principal investigator was from Poland, and the Polish ethics committee was the Bioethics Committee at the Poznan University of Medical Sciences, resolution no. 17/02 of 5 January 2017.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Viscusi, E.R., Langford, R., Morte, A. et al. Safety of Co-Crystal of Tramadol-Celecoxib (CTC) in Patients with Acute Moderate-to-Severe Pain: Pooled Analysis of Three Phase 3 Randomized Trials. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00655-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40122-024-00655-w