Abstract
Introduction
Several factors may lead to increased postoperative pain sensitivity, of which remifentanil-induced hyperalgesia (RIH) is one of the main factors. High-dose remifentanil exposure during anesthesia may induce RIH. Esketamine may prevent RIH by antagonizing N-methyl-d-aspartate (NMDA) receptors, thereby reducing the postoperative pain sensitivity. This study examined the effects of different esketamine doses on pain sensitivity in patients undergoing thyroidectomy and determined the optimal dose.
Methods
This study included 117 patients who received elective thyroidectomy. They were randomized into four groups: saline group (group C), esketamine 0.2 mg·kg−1 group (group RK1), esketamine 0.4 mg·kg−1 group (group RK2), and esketamine 0.6 mg·kg−1 group (group RK3). Five minutes before anesthesia induction, the same volume of study drugs were injected respectively in groups C, RK1, RK2, and RK3. Remifentanil was pumped at the same rate of 0.3 µg·kg−1·min−1 during surgery to ensure uniformity. This study’s primary outcomes were the mechanical pain thresholds measured before surgery, as well as at 30 min, 6 h, 24 h, and 48 h after surgery. Hyperalgesia, rescue analgesia, numerical rating scale (NRS) score, and adverse reactions were recorded.
Results
Compared with baseline, the mechanical pain threshold was significantly decreased in group C [(94.67 ± 22.85) versus (112.00 ± 36.62) versus (161.33 ± 53.28) g, P < 0.001 at 30 min, P < 0.001 at 6 h] and group RK1 [(102.86 ± 24.17) versus (114.29 ± 41.05) versus (160.00 ± 54.98) g, P < 0.001 at 30 min, P < 0.001 at 6 h] around the surgical incision, and in group C [(112.00 ± 31.78) versus (170.67 ± 56.26) g, P < 0.001 at 30 min, (118.67 ± 34.42) versus (170.67 ± 56.26) g, P = 0.001 at 6 h] and group RK1 [(114.29 ± 45.17) versus (175.71 ± 54.80) g, P = 0.001 at 30 min, (121.43 ± 38.46) versus (175.71 ± 54.80) g, P = 0.002 at 6 h] on the forearm at 30 min and 6 h after surgery; compared with group C, the mechanical pain threshold was higher in group RK2 [(142.76 ± 50.06) versus (94.67 ± 22.85) g, P < 0.001 at 30 min, (145.52 ± 49.83) versus (112.00 ± 36.62) g, P < 0.001 at 6 h] and group RK3 [(140.00 ± 40.68) versus (94.67 ± 22.85) g, P < 0.001 at 30 min, (150.67 ± 56.50) versus (112.00 ± 36.62) g, P = 0.010 at 6 h] around the surgical incision, and in group RK2 [(149.66 ± 39.50) versus (112.00 ± 31.78) g, P = 0.006 at 30 min, (156.55 ± 47.23) versus (118.67 ± 34.42) g, P = 0.005 at 6 h] and group RK3 [(145.33 ± 51.18) versus (112.00 ± 31.78) g, P = 0.018 at 30 min, (154.67 ± 47.54) versus (118.67 ± 34.42) g, P = 0.008 at 6 h] on the forearm at 30 min and 6 h after surgery. Group RK3 had more glandular secretions than the other three groups (P = 0.042).
Conclusions
Intravenous injection of esketamine 0.4 mg·kg−1 before anesthesia induction is a suitable dose to reduce pain sensitivity in patients undergoing thyroidectomy without increasing adverse reactions. However, future research needs to be extended to other populations.
Trial Registration
Registered at the Chinese Clinical Trials Registry http://www.chictr.org.cn/ (09/06/2022, ChiCTR-2200060741).
Similar content being viewed by others
Why carry out this study? |
The pain after thyroidectomy is mild-to-moderate and can last about 48 hours. Remifentanil-induced hyperalgesia (RIH) is one of the main factors that increased postoperative pain sensitivity. Poor pain control in the early postoperative period may delay recovery and develop chronic pain. Acute pain in humans can also be measured by Von Frey filaments. |
RIH has been found to be associated with N-methyl-d-aspartate (NMDA) receptors activated by remifentanil. Esketamine is an anesthetic adjuvant, which has analgesic effect and antagonistic NMDA receptor effect. However, there are few studies on whether esketamine reduces postoperative pain sensitivity by preventing RIH. |
What was learned from the study? |
In this study, esketamine was found to reduce pain sensitivity after thyroidectomy with little effect on the hemodynamics of patients. |
Therefore, intravenous injection of esketamine 0.4 mg·kg-1 before anesthesia induction is a suitable dose to reduce pain sensitivity in patients undergoing thyroidectomy. |
Introduction
Remifentanil is a µ-opioid receptor agonist commonly used in general anesthesia due to its rapid onset and short half-life [1]. However, clinical studies have found that remifentanil-induced hyperalgesia (RIH) is associated with an increase in pain sensitivity after remifentanil administration during surgery [2, 3]. RIH has been found to be associated with N-methyl-d-aspartate (NMDA) receptors activated by remifentanil [4, 5], as well as central sensitization [6]. Continuous infusion of 0.3 µ·kg−1·min−1 remifentanil for intraoperative analgesia could decrease the mechanical pain threshold and induce RIH [7, 8]. RIH leads to poor early postoperative analgesia treatment and is a risk factor for chronic pain [9]. Therefore, reducing RIH has positive clinical significance in reducing the occurrence of postoperative chronic pain. Quantitative sensory test (QST) was used to identify altered pain sensitivity and quantify hyperalgesia in surgical patients [10]. Pain sensitivity, which was measured using the QST, can predict the development of acute postoperative pain [11].
Subanesthetic ketamine can be used as an adjunct during general anesthesia to reduce postoperative pain and opioid consumption [12]. Ketamine attenuates RIH by modulating NMDA receptor activity [13]. Esketamine, an optically active isomer of ketamine [14], has approximately twice the affinity of ketamine for NMDA receptor [15, 16]. Therefore, subclinical doses of esketamine may reduce side effects, such as nightmares, delirium, and agitation [17]. Previous studies have found that remifentanil infusion during thyroid surgery is associated with higher postoperative pain and postoperative analgesic drug demand [18, 19]. The effect of a subanesthetic dose of esketamine on RIH in patients undergoing thyroidectomy has not been explored, and the optimal dose of esketamine to alleviate RIH remains unclear.
This study was conducted to confirm whether intravenous different doses of esketamine, before induction of anesthesia, would reduce hyperalgesia after remifentanil infusion in patients undergoing thyroidectomy, and to confirm the optimal dose.
Methods
Study Design and Settings
This study was a double-blind randomized controlled clinical trial. It was performed at the First Affiliated Hospital of Zhengzhou University from 9 June 2022 to 31 August 2022. The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University (2021-KY-0858–002), which was registered at www.chictr.org.cn (no. ChiCTR-2200060741, registration data June 09, 2022). This study protocol was performed according to CONSORT guidelines. Before surgery, all participants signed an informed consent form.
Participants
This study enrolled 117 patients. Inclusion criteria: patients scheduled for elective thyroidectomy under general anesthesia, aged 18–60 years, any sex, American Society of Anesthesiologists (ASA) physical status I or II, and body mass index (BMI) of 18–30 kg·m−2. Exclusion criteria: history of thyroid surgery or hyperthyroidism, hypertension or coronary heart disease, chronic pain, opioid drug abuse, allergy to esketamine, renal or liver dysfunction, psychiatric disorders, neurological disease, or refusal to participate in the study.
Based on the random number, all participants were randomly divided at a ratio of 1:1:1:1 into four groups: C, RK1, RK2, RK3. We used the SPSS 26.0 software package (IBM SPSS, Chicago, Illinois, USA) to generate random numbers. An independent nurse divided the patients according to the recruitment sequence and the random number, prepared the study drug, and provided it to the anesthesiologist. The anesthesiologists and investigators responsible for postoperative follow-up were blinded to the group assignments.
Von Frey filament (Stoelting, Wood Dale, IL, USA) is a classic noninvasive tool and widely used in laboratory and clinical studies [20]. Von Frey was used to measure the pain threshold to determine the occurrence of hyperalgesia in this study. All Von Frey filament tests were performed by the same investigator. The measurement environment was quiet and the patient lay flat and relaxed. The tip of the Von Frey filament was placed in contact with the skin surface at right angles, and pressure was applied to bend the filament for 2 s. Pressure was applied from the 60 g filament, when the patient’s sensation changed from light touch to tingling, it was the mechanical pain threshold of the patient. The measurements were repeated at the same position at intervals of 10 s. Mechanical pain threshold around thyroid incision: the mean value was measured 2 cm below the incision midpoint and at both ends of the incision, and non-dominant forearm: the mean value was measured on the skin at 3, 6, and 9 cm from the anterior elbow crease on the medial forearm [21, 22]. A lower mechanical pain threshold indicates greater sensitivity to pain and a lower postoperative pain threshold indicates hyperalgesia.
Protocol
All patients fasted routinely before surgery. Electrocardiography (ECG), noninvasive blood pressure (NBP), oxygen saturation (SpO2), and BIS pectrum index (BIS) were routinely monitored and recorded every 3 min. All patients were injected with 10 ml study drug 5 min before anesthesia induction.
Anesthesia induction: All patients received intravenous propofol (21,121,531, Yangzijiang Pharmaceutical Group Co., Ltd.) 2 mg·kg−1 and remifentanil (20A02171, Yichang Renfu Pharmaceutical Co., Ltd.) 1.5 µg·kg−1. Rocuronium (EA2194, Zhejiang Xianju Pharmaceutical Co., Ltd.) 0.6 mg·kg−1 was injected after the patient lost consciousness. Mechanical ventilation was provided following endotracheal intubation. Anesthesia maintenance: 0.3 µg·kg−1·min−1 remifentanil was continuously pumped intravenously, and 1.5% sevoflurane (22,060,531, Jiangsu Hengrui Medicine Co., Ltd.) was inhaled. During the surgery, the anesthesiologist adjusted the inhalation concentration of sevoflurane on the basis of vital signs and BIS target value of 40–60. Heart rate and mean arterial pressure of the patients were maintained within 20% of baseline. Vasoactive drugs were administered when necessary. Palonosetron (210830CA, Jiangsu Hengrui Medicine Co., Ltd) 0.25 mg was intravenously injected 30 min after the surgery began. Remifentanil and sevoflurane were stopped immediately after surgery. All patients were resuscitated in the postanesthesia care unit (PACU). The nurse assessed the patient’s state of consciousness by calling or patting the shoulder every 3 mins. When the patient was fully awake, swallowing and cough reflexes recovered completely, and breathing air SpO2 was greater than 90%, the endotracheal tube was extubated. Extubation time was recorded. If the NRS score was greater than 4 or the patient required rescue analgesia, flurbiprofen axetil 50 mg was administered.
Outcome Measures
The mechanical pain thresholds measured before surgery as well as at 30 min, 6 h, 24 h, and 48 h after surgery were this study's primary outcomes. NRS scores, the incidence of postoperative hyperalgesia, rescue analgesia, perioperative ephedrine and atropine use, and adverse reactions, including delirium, hallucinations, nausea ,and vomiting were recorded. Compared with the baseline, the postoperative mechanical pain threshold was significantly decreased, and we defined this as hyperalgesia.
Sample Size Calculation
The sample size was determined by the primary outcome of our previous study. The mean ± standard deviation (SD) mechanical pain threshold in groups C, RK1, RK2, and RK3 were 95.6 ± 22.4 g, 101.7 ± 24.5 g, 140.3 ± 50.1 g, and 141.0 ± 41.4 g around the surgical incision 30 min after surgery. We calculated a sample size of 21 participants per group. The significance level was 0.05 (α = 0.05), and the power was 90%. Assuming a 30% attrition rate, we calculated that 30 participants would be required for each group. This study used the PASS 15.0 software (Stata Corp. LP, College Station, Texas, USA) to calculate the sample size.
Statistical Analysis
Kolmogorov–Smirnov test and histograms were used to verify the normal distribution of the data. Quantitative variables were presented as mean ± SD and analyzed using Student's t-test, or median [interquartile range (IQR)] and analyzed using the Mann–Whitney U test. Categorical variables, specified as frequency (f) and number (%), were compared using Pearson’s chi-squared test or Fisher’s exact test. Mechanical pain thresholds were analyzed using two-way repeated measures ANOVA with Bonferroni post hoc comparison. NRS scores were analyzed using the Kruskal–Wallis test. We also used post hoc pairwise comparisons of NRS scores of the four groups at each time point after surgery, and the significance criterion was P < 0.0083 after Bonferroni correction. Hyperalgesia incidence, rescue analgesic requirement, and adverse reactions was compared among the four groups using the Pearson’s chi-squared test or Fisher’s exact test. A P-value < 0.05 was considered statistically significant.
Results
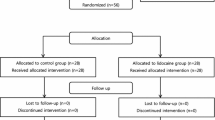
This study assessed 120 patients from June to August 2022. Two patients who were transferred to other departments 24 h after surgery were excluded, and one patient refused to cooperate and withdrew from the trial. Therefore, 117 patients (group C = 30, group RK1 = 28, group RK2 = 29, and group RK3 = 30) were analyzed (Fig. 1).
Patient Features
As shown in Table 1, patient age (P = 0.624), sex (P = 0.816), BMI (P = 0.330), operation time (P = 0.509), anesthesia time (P = 0.437), and remifentanil total dose (P = 0.829) were not significant differences among the four groups. The time from the end of surgery to endotracheal tube removal was defined as extubation time. The extubation time in group RK3 [14.7, 95% confidence interval (CI) 11.5–17.9] was significantly longer than that in groups C (8.4, 95% CI 6.4–10.4), RK1 (8.9, 95% CI 6.3–11.7), and RK2 (11.1, 95% CI 8.5–13.7) (Table 1; P = 0.001, 0.002, and 0.044, respectively). The PACU stay time in group RK3 (32.9, 95% CI 30.4–35.5) was significantly longer than that in groups C (28.7, 95% CI 26.2–31.3), RK1 (28.3, 95% CI 25.3–31.3), and RK2 (29.1, 95% CI 26.9–31.3) (Table 1; P = 0.018, 0.011, and 0.032, respectively).
Mechanical Pain Threshold
Around the surgical incision: the four groups did not differ significantly before surgery; group RK1 was not significantly different from group C after surgery (Fig. 2A), group RK2 [Fig. 2A; (142.8 ± 50.1) versus (94.7 ± 22.9) g, P < 0.001 at 30 min, (145.5 ± 49.8) versus (112.0 ± 36.6) g, P < 0.001 at 6 h] and group RK3 [Fig. 2A; (140.0 ± 40.7) versus (94.7 ± 22.9) g, P < 0.001 at 30 min; (150.7 ± 56.5) versus (112.0 ± 36.6) g, P = 0.010 at 6 h] were higher than group C at 30 min and 6 h after surgery. Group C [Fig. 2A; (94.7 ± 22.9) versus (112.0 ± 36.6) versus (161.3 ± 53.3) g, P < 0.001 at 30 min, P < 0.001 at 6 h] and group RK1 [Fig. 2A; (102.9 ± 24.2) versus (114.3 ± 41.1) versus (160.0 ± 54.9) g, P < 0.001 at 30 min, P < 0.001 at 6 h] were lower than the baseline at 30 min and 6 h after surgery. Groups RK2 and RK3 have no significant difference between pre- and postoperative; no significant differences were found among the four groups or compared with the baseline 24 and 48 h after surgery (Fig. 2A).
On the forearm: the four groups did not differ significantly before surgery; groups RK1 and C showed no significant difference after surgery (Fig. 2B). Group RK2 [Fig. 2B; (149.7 ± 39.5) versus (112.0 ± 31.8) g, P = 0.006 at 30 min; (156.6 ± 47.2) versus (118.7 ± 34.4) g, P = 0.005 at 6 h] and group RK3 [Fig. 2B; (145.3 ± 51.2) versus (112.0 ± 31.8) g, P = 0.018 at 30 min; (154.7 ± 47.5) versus (118.7 ± 34.4) g, P = 0.008 at 6 h] were higher than group C at 30 min and 6 h after surgery. Group C [Fig. 2B; (112.0 ± 31.8) versus (170.7 ± 56.3) g, P < 0.001 at 30 min; (118.7 ± 34.4) versus (170.7 ± 56.3) g, P = 0.001 at 6 h] and group RK1 [Fig. 2B; (114.3 ± 45.2) versus (175.7 ± 54.8) g, P = 0.001 at 30 min; (121.4 ± 38.5) versus (175.7 ± 54.8) g, P = 0.002 at 6 h] were significantly decreased compared with the baseline at 30 min and 6 h after surgery. Groups RK2 and RK3 showed no significant difference between pre- and postoperative. No differences were found among the four groups or compared with the baseline 24 and 48 h after surgery (Fig. 2B).
Hyperalgesia Incidence
At 30 min and 6 h after surgery, hyperalgesia in group C was higher than that in group RK3 around the surgical incision [Table 2; 19 (63.3%) versus 2 (6.7%), P = 0.001 at 30 min; 17 (60.7%) versus 1 (3.6%), P = 0.001 at 6 h] and on the forearm [Table 2; 15 (50.0%) versus 2 (6.7%), P = 0.006 at 30 min; 15 (53.6%) versus 3 (10.7%), P = 0.002 at 6 h], and was higher than that in group RK2 around the surgical incision [Table 2; 19 (63.3%) versus 5 (16.7%), P = 0.001 at 30 min; 17 (60.7%) versus 3 (10.7%), P = 0.002 at 6 h] and on the forearm [Table 2; 15 (50.0%) versus 3 (10.0%), P = 0.008 at 30 min; 15 (53.6%) versus 4 (14.3%), P = 0.006 at 6 h]. No significant difference was found among the four groups 24 h and 48 h after surgery (Table 2). Significant differences in hyperalgesia between groups C and RK1, and between groups RK2 and RK3 were found (Table 2).
NRS score, Incidence of Rescue Analgesia, Adverse Reactions
The NRS score in group RK3 was lower than that in groups C (P = 0.002), RK1 (P < 0.001), and RK2 (P = 0.003) at 30 min after surgery, and was lower than that in groups C (P = 0.002) and RK1 (P = 0.004) at 6 h after surgery. Groups C, RK1, and RK2 did not differ significantly at 30 min and 6 h after surgery. The four groups showed no significant differences in NRS scores at 24 and 48 h after surgery (Table 3; P = 0.081 at 24 h and P = 0.407 at 48 h).
The four groups did not differ significantly in rescue analgesia (Table 4; P = 0.455).
Group RK3 had more glandular secretions (P = 0.042). Neither perioperative ephedrine and atropine use nor postoperative adverse reactions, including delirium, hallucinations, nausea, and vomiting, showed significant differences among the four groups (Table 4).
Discussion
Our results showed that remifentanil infused at a continuous rate of 0.3 µg·kg−1·min−1 during thyroid surgery could increase postoperative hyperalgesia. This trial aimed to determine the effects of esketamine on pain sensitivity after remifentanil infusion in thyroidectomy patients. According to our data, intravenous administration of esketamine 5 min before anesthesia induction could increase the postoperative mechanical pain threshold and decrease the hyperalgesia incidence, with an optimal dose of 0.4 mg·kg−1.
Surgical procedures lead to postoperative pain and increased opioid use. Postoperative pain is a complex process that is mainly affected by central sensitization, surgical injury, and release of inflammatory factors [23]. In addition to surgery, another external factor affecting postoperative pain is opioid-induced hyperalgesia. Opioid-naive healthy human volunteers develop RIH after remifentanil infusion [24]. The mechanism of RIH may be related to the upregulation and activation of NMDA receptors in the spinal cord, leading to central sensitization [5, 25]. In previous studies, Von Frey filaments can also be used to measure acute pain in humans [26]. The mechanical pain threshold at 30 min and 6 h after surgery in group C was significantly lower than the baseline, indicating hyperalgesia occurred, and this result is in line with previous studies [27, 28]. Intraoperative opioids and surgical injuries may jointly lead to postoperative hyperalgesia. The reasons for lower mechanical pain thresholds around surgical incisions include RIH and tissue trauma stimulation, while those on the forearm may be less associated with tissue trauma, suggesting that esketamine may reduce the mechanical pain threshold in normal tissue sites by inhibiting RIH effects.
Central NMDA receptors play an important role in central sensitization and hyperalgesia [29] and contribute to RIH [30]. Animal studies have found that blocking NMDA receptors can prevent the development of opioid-related pain sensitivity [31]. NMDA receptor antagonists can be used clinically to regulate opioid-induced hyperalgesia [20]. As ketamine can reduce NMDA receptor-mediated secondary hyperalgesia, low-dose intravenous ketamine can be used as an adjuvant drug for acute and chronic postoperative pain management [32]. Esketamine has a higher clearance rate in vivo and theoretically a lower incidence of side effects [33]. Therefore, esketamine has great research prospects and significance for the prevention and treatment of RIH. This study showed that intravenous administration of esketamine 0.4 mg·kg−1 and 0.6 mg·kg−1 before anesthesia induction increased mechanical pain thresholds and decreased the incidence of hyperalgesia at 30 min and 6 h postoperatively. This may be related to the long-term antagonistic effect of esketamine on the NMDA receptors. In addition, norketamine is the active metabolite of esketamine conversion in vivo, and its anesthetic effect is 1:5 to 1:3 that of esketamine, with a longer elimination half-life, which may also be related to the longer analgesic time of esketamine [34, 35].
Quantitative Sensory Testing (QST) is an objective and accurate measurement method that can identify changes in pain sensitivity in patients [36]. Studies have shown that pain sensitivity measured by QST can improve its predictive value for postoperative pain development [37]. The Von Frey filament is a well-repeatable QST detection method, which is more sensitive and objective for sensory testing of pain and is commonly used to measure mechanical pain thresholds in clinical studies [20]. The pain indicators in this study included mechanical pain threshold, NRS score, and incidence of rescue analgesia. In the present study, esketamine produced a dose-dependent anti-hyperalgesia effect. Except that NRS score in group RK3 was lower than group RK2 at 30 min after surgery, groups RK3 and RK2 did not differ significantly at other time points after surgery. Groups RK3 and RK2 did not differ significantly in rescue analgesia and hyperalgesia after surgery. The results indicated that 0.6 mg·kg−1 and 0.4 mg·kg−1 esketamine had similar analgesic effects in the early period of postoperation. Patients who received 0.6 mg·kg−1 esketamine intravenously had longer extubation time and PACU stay time, and more adverse reactions such as increased gland secretion. Therefore, it is speculated that intravenous injection of 0.4 mg·kg−1 esketamine before anesthesia induction may be a suitable dose to prevent RIH.
This study mainly evaluated the mechanical pain threshold in patients with thyroid cancer after surgery, which is a type of spontaneous pain. Pain after thyroid surgery is confined to the anterior neck, and the intensity of pain is moderate to mild. It is not aggravated by exercise and can be quickly relieved by low-dose analgesics [38]. Esketamine was effective within 30 s after intravenous injection, the duration of action was 30–45 min, and the clearance half-life was 155 ± 42 min. In this study, esketamine was administered as a single intravenous injection 5 min before anesthesia induction. Although the half-life of esketamine is short, this study showed that the postoperative analgesic effect of esketamine is greater than 6 h. This phenomenon may be explained by the preemptive analgesic effect of prophylactic analgesics before the onset of traumatic conduction and central nervous system hypersensitivity [39]. The degree of analgesic effect of a single administration of esketamine is influenced by the type of surgery, and continuous intravenous administration may be required to provide superior postoperative analgesia in more painful surgeries. We observed no serious adverse effects that required withdrawal from the trial throughout the study. Patients using vasoactive drugs showed no significant differences among the four groups, indicating that low-dose esketamine had little effect on the perioperative hemodynamics.
Our study had some limitations. First, we only measured the mechanical pain threshold, and the temperature and electrical pressure pain thresholds could be measured in the future. Second, no chronic postoperative pain was observed. Third, there are variables in the human patient population, including gender, red hair, and many other factors. Therefore, the findings of the study are limited to generalization. Further research is required to address these questions.
Conclusions
Intravenous injection of esketamine 0.4 mg·kg−1 before anesthesia induction is a suitable dose to reduce pain sensitivity in patients undergoing thyroidectomy without increasing adverse reactions. However, future research needs to be extended to other populations.
References
Brown EN, Pavone KJ, Naranjo M. Multimodal General Anesthesia: Theory and Practice. Anesth Analg. 2018; 127(5):1246–1258. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6203428/.
Santonocito C, Noto A, Crimi C, Sanfilippo F. Remifentanil-induced postoperative hyperalgesia: current perspectives on mechanisms and therapeutic strategies. Local Reg Anesth. 2018; 11: 15–23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5898588/.
Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Remifentanil-acute opioid tolerance and opioid-induced hyperalgesia: a systematic review. Am J Ther. 2015; 22(3):e62- 74. https://pubmed.ncbi.nlm.nih.gov/25830866/.
Low Y, Clarke CF, Huh BK. Opioid-induced hyperalgesia: a review of epidemiology, mechanisms and management. Singapore Med J. 2012; 53(5):357–60. https://pubmed.ncbi.nlm.nih.gov/22584979/.
Su L, Bai Xq, Niu Tx, Zhuang Xq, Dong Bb, Wang Gl, Yu Yh. P2Y1 purinergic receptor inhibition attenuated remifentanil-induced postoperative hyperalgesia via decreasing NMDA receptor phosphorylation in dorsal root ganglion. Brain Res Bull. 2021; 177:352–362. https://pubmed.ncbi.nlm.nih.gov/34653560/.
Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011; 152(3 Suppl):S2–15. https://pubmed.ncbi.nlm.nih.gov/20961685/.
Zhang L, Shu R, Zhao Q, Li Y, Yu Y, Wang G. Preoperative butorphanol and flurbiprofen axetil therapy attenuates remifentanil-induced hyperalgesia after laparoscopic gynaecological surgery: a randomized double-blind controlled trial. Br J Anaesth. 2016; 117(4):504- 511. https://pubmed.ncbi.nlm.nih.gov/28077539/.
Yu EHY, Tran DH, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: Short term gain, long-term pain? Anaesthesia. 2016; 71(11):1347–1362. https://pubmed.ncbi.nlm.nih.gov/27734470/.
Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000; 93(4):1123–33. https://pubmed.ncbi.nlm.nih.gov/11020770/.
Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009; 10(6):556–72. https://pubmed.ncbi.nlm.nih.gov/19380256/.
Werner MU, Mjöbo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anesthesiology. 2010; 112(6):1494–502. https://pubmed.ncbi.nlm.nih.gov/20460988/.
Wang Xm, Lin C, Lan L, Liu Jc. Perioperative intravenous S- ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J Clin Anesth. 2021; 68:110071. https://pubmed.ncbi.nlm.nih.gov/33007645/.
Choi E, Lee H, Park HS, Lee GY, Kim YJ, Baik HJ. Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2015; 68(5):476–80. https://pubmed.ncbi.nlm.nih.gov/26495058/.
Jelen LA, Young AH, Stone JM. Ketamine: A tale of two enantiomers. J Psychopharmacol. 2021; 35(2):109- 123. https://pubmed.ncbi.nlm.nih.gov/33155503/
Ithnin FB, Tan DJA, Xu XL, Tan CH, Sultana R, Sng BL. Low-dose S+ketamine in target controlled intravenous anaesthesia with remifentanil and propofol for open gynaecological surgery: A randomised controlled trial. Indian J Anaesth. 2019; 63(2):126- 133. https://pubmed.ncbi.nlm.nih.gov/30814750/.
Wang J, Huang J, Yang S, Cui C, Ye L, Wang SY, Yang GP, Pei Q. Pharmacokinetics and safety of Esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019; 13:4135–4144. https://pubmed.ncbi.nlm.nih.gov/31827320/.
Muller J, Pentyala S, Dilger J, Pentyala S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016; 6(3):185- 92. https://pubmed.ncbi.nlm.nih.gov/27354907/.
Tharakan T, Jiang S, Fastenberg J, Ow TJ, Schiff B, Smith RV, Mehta V. Postoperative Pain Control and Opioid Usage Patterns among Patients Undergoing Thyroidectomy and Parathyroidectomy. Otolaryngol Head Neck Surg. 2019; 160(3):394–401. https://pubmed.ncbi.nlm.nih.gov/30324865/.
James X. Wu, Melissa Assel, Andrew Vickers, Anoushka M. Afonso, Rebecca S Twersky, Brett A. Simon, Marc A. Cohen, Elizabeth F. Rieth, Jennifer R. Cracchiolo. Impact of Intraoperative Remifentanil on Postoperative Pain and Opioid Use in Thyroid Surgery. J Surg Oncol. 2019; 120(8):1456–1461. https://pubmed.ncbi.nlm.nih.gov/31680250/.
Shu R, Zhang L, Zhang H, Li Y, Wang C, Su L, Zhao H, Wang G. NMDA Receptor Modulates Spinal Iron Accumulation Via Activating DMT1(-)IRE in Remifentanil-Induced Hyperalgesia. J Pain. 2021; 22(1):32–47. https://pubmed.ncbi.nlm.nih.gov/32574785/
Chen Y, Yao Y, Wu Y, Dai D, Zhao Q, Qiu L. Transcutaneous electric acupoint stimulation alleviates remifentanil-induced hyperalgesia in patients undergoing thyroidectomy: a randomized controlled trial. Int J Clin Exp Med. 2015;8(4):5781–7. https://pubmed.ncbi.nlm.nih.gov/26131165/.
Mailloux C, Beaulieu LD, Wideman TH, Massé-Alarie H. Within-session test-retest reliability of pressure pain threshold and mechanical temporal summation in healthy subjects. PLoS One. 2021; 16(1): e0245278. https://pubmed.ncbi.nlm.nih.gov/33434233/
Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, De Koninck Y. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl-homeostasis. Nat Neurosci. 2013;16(2):183-92. https://pubmed.ncbi.nlm.nih.gov/23292683/.
Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103(1):147–55. https://pubmed.ncbi.nlm.nih.gov/15983467/.
Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. https://pubmed.ncbi.nlm.nih.gov/30983589/.
Schneider T, Luethi J, Mauermann E, Bandschapp O, Ruppen W. Pain Response to Open Label Placebo in Induced Acute Pain in Healthy Adult Males. Anesthesiology. 2020; 132(3):571–580. https://pubmed.ncbi.nlm.nih.gov/31809325/
de Hoogd S, Valkenburg AJ, van Dongen EPA, Daeter EJ, van Rosmalen J, Dahan A, Tibboel D, Knibbe CAJ. Short-and long-term impact of remifentanil on thermal detection and pain thresholds after cardiac surgery: A randomised controlled trial. Eur J Anaesthesiol. 2019;36(1):32–39. https://pubmed.ncbi.nlm.nih.gov/30211725/.
de Hoogd S, Ahlers SJ, van Dongen EP, van de Garde EM, Hamilton-Ter Brake TA, Dahan A, Tibboel D, Knibbe CA. Is Intraoperative Remifentanil Associated With Acute or Chronic Postoperative Pain After Prolonged Surgery? An Update of the Literature. Clin J Pain. 2016;32(8):726–35. https://pubmed.ncbi.nlm.nih.gov/26626296/.
Cichon J, Blanck TJJ, Gan WB, Yang G. Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci. 2017;20(8):1122–1132. https://pubmed.ncbi.nlm.nih.gov/28671692/.
Wang C, Li Y, Wang H, Xie K, Shu R, Zhang L, Hu N, Yu Y, Wang G. Inhibition of DOR prevents remifentanil induced postoperative hyperalgesia through regulating the trafficking and function of spinal NMDA receptors in vivo and in vitro. Brain Res Bull. 2015;110:30–9. https://pubmed.ncbi.nlm.nih.gov/25498394/.
Li T, Wang H, Wang J, Chen Y, Yang C, Zhao M, Wang G, Yang Z. Annexin 1 inhibits remifentanil-induced hyperalgesia and NMDA receptor phosphorylation via regulating spinal CXCL12/CXCR4 in rats. Neurosci Res. 2019; 144: 48–55. https://pubmed.ncbi.nlm.nih.gov/30120960/.
Ekhtiari S, Bhandari M. Cochrane in CORR(R): perioperative intravenous ketamine for acute postoperative pain in adults. Clin Orthop Relat Res. 2019; 477(11):2411- 2417. https://pubmed.ncbi.nlm.nih.gov/30570761/.
Eberl S, Koers L, van Hooft JE, de Jong E, Schneider T, Hollmann MW, Preckel B. Sedation with propofol during ERCP: is the combination with esketamine more effective and safer than with alfentanil? Study protocol for a randomized controlled trial. Trials. 2017; 18(1): 472. https://pubmed.ncbi.nlm.nih.gov/29020995/.
Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol Rev. 2018; 70(3):621–660. https://pubmed.ncbi.nlm.nih.gov/29945898/.
Ye L, Xiao X, Zhu L. The comparison of etomidate and propofol anesthesia in patients undergoing gastrointestinal endoscopy: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech. 2017; 27(1):1–7. https://pubmed.ncbi.nlm.nih.gov/28079763/
Mücke M, Cuhls H, Radbruch L, Baron R, Maier C, Tölle T, Treede RD, Rolke R. Quantitative sensory testing (QST). English version. Schmerz. 2021; 35(Suppl 3):153–160. https://pubmed.ncbi.nlm.nih.gov/26826097/.
Treede RD. The role of quantitative sensory testing in the prediction of chronic pain. Pain. 2019; 160 Suppl 1:S66-S69. https://pubmed.ncbi.nlm.nih.gov/31008852/
Romero Arenas MA, Uhlmann RA, Postevka E, Wang X, Reinhart HA, Snyder SK. Multimodal analgesia after thyroid or parathyroid surgery: A randomized controlled trial. Surgery. 2021; 169(3):508–512. https://pubmed.ncbi.nlm.nih.gov/32977975/.
Byrne K, Smith C. Preemptive Analgesia: An Unobtainable Goal? J Cardiothorac Vasc Anesth. 2019; 33(2):460–461. https://pubmed.ncbi.nlm.nih.gov/30217585/
Acknowledgements
We thank all the participants of the study, including patients and our colleagues.
Funding
This study was supported by the National Natural Science Foundation of China (82001187 and 82071240). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Wei Zhang, Yan-ling Ren, Fei Xing, and Jing-jing Yuan designed the study. Li-ning Zhu and Yan-ling Ren recruited patients. Yan-ling Ren performed statistical processing and wrote the manuscript. Fei Xing and Jing-jing Yuan revised the manuscript. All authors are aware of and responsible for the research data. All authors read and approved the manuscript in its final version.
Disclosures
Yan-ling Ren, Jing-jing Yuan, Fei Xing, Li-ning Zhu, and Wei Zhang have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2021-KY-0858–002) and was registered at the Chinese Clinical Trials Registry http://www.chictr.org.cn/ (09/06/2022, ChiCTR-2200060741). The study protocol followed the CONSORT guidelines. Written informed consent was obtained from each participant.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ren, Yl., Yuan, Jj., Xing, F. et al. Effects of Different Doses of Esketamine on Pain Sensitivity of Patients Undergoing Thyroidectomy: A Randomized Controlled Trial. Pain Ther 12, 739–750 (2023). https://doi.org/10.1007/s40122-023-00488-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-023-00488-z