Abstract
Cocoa has been reported to have medicinal properties. It contains a wide range of phytochemicals, including polyphenols, which have been shown to exert anti-inflammatory and antioxidant actions, and also to have a positive effect on pain. Other components of cocoa might be able to positively influence pain perception through various mechanisms. Despite encouraging results from preclinical studies, there is a lack of evidence of antinociceptive effects of cocoa from clinical trials in humans. Further research is needed to better identify the active principles in cocoa, to understand the underlying mechanisms of action, and to establish efficacy in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cocoa and its derived products have been described as potential medicines. |
Cocoa contains a wide range of phytochemicals. The main constituents are methylxanthines and flavan-3-ols. |
Different anti-inflammatory and anti-nociceptive effects have been attributed to compounds isolated from cocoa and other plants. |
We stated the possible relationship between cocoa intake and anti-inflammatory anti-nociceptive effects. |
Introduction
Pain is a global public health problem. Efficacious pain treatment relies on effective drugs to ensure an optimal analgesia with minimal adverse effects. Because of the adverse reactions, the physical dependence, and the tendency to abuse observed with the available drugs, it seems necessary to look for new molecules [1].
Since the 17th century, cocoa has been reported to have medicinal properties and has been used for various purposes, including the treatment of angina and heart pain [2]. A wide variety of active compounds are present in cocoa beans. Among them, methylxanthines and flavan-3-ols (e.g., proanthocyanidins) are well represented. There are also other classes of polyphenols (such as flavonols, anthocyanins, stilbenoids, and phenolic acid derivatives), amides/amines, and alkaloids [3].
According to this, currently, catechin polyphenols, anthocyanidins, and proanthocyanidins are considered the main cocoa compounds with anti-inflammatory and anti-nociceptive effects [4]. Therefore, this review aims to resume the evidence regarding the anti-inflammatory and anti-nociceptive effects of cocoa flavonoids. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Cocoa and Pain: Experimental Studies
Furthermore, it has recently been observed that the activation of trigeminal neurons and the expression of proteins involved in nociception in the ganglion and spinal cord were inhibited by cocoa [5, 6]. A study conducted by Bowden et al. [6] showed an inhibition of neurogenic inflammatory orofacial pain in rats fed with a diet rich in cocoa.
Further, a study conducted by Cady et al. [7] showed how dietary cocoa administration was able to increase the circulating levels of different peptides with anti-inflammatory and anti-nociceptive properties and on the other hand counteracted the inflammation by inhibiting the expression of pro-phlogogenic proteins and neuronal sensitization [7].
Specifically, male Sprague–Dawley rats were fed either a control diet or a cocoa-enriched diet prior to injection of complete Freund’s adjuvant (CFA) into the temporomandibular joint capsule. CFA contains heat-killed mycobacteria and, when injected, induces a painful long-lasting inflammatory status, thus promoting prolonged activation of trigeminal ganglion neurons and glia. Cocoa stimulated an increase in the glial expression of the glutamate transport protein GLAST, which removes the excitatory neurotransmitter glutamate from the external environment around second-order neurons. In the spinal trigeminal nucleus, cocoa was also able to decrease the expression of calcitonin gene-related peptide (CGRP). In this regard, several studies suggest how, at the glial cell level, the release of CGRP is able to increase cellular activation, resulting in an increased production of proinflammatory molecules. Similarly, CGRP was also shown to be able to increase the synthesis of protein kinase A (PKA) and purinoreceptor (P2X) 3, enhancing the sensitization of nociceptive neurons also at the level of the spinal glia. Cocoa repressed the CFA-dependent increase in the levels of P2X and PKA. Another important finding from this study was that dietary cocoa increased the expression of the anti-inflammatory protein MAP kinase phosphatases 1 (MKP-1). MKP regulate the cellular responses mediated by the mitogen-activated protein kinase (MAPK) and are involved in inflammatory and nociceptive processes. Furthermore, cocoa was able to counteract the inflammation induced by cytokines dependent by CFA and repress CFA-induced expression of glial-fibrillary-associated protein (GFAP) and OX-42, respectively, markers of astrocytes and microglia activation [7].
These results are in agreement with those obtained in another study by Cady et al. [8], in which rats fed a diet enriched in cocoa showed elevated basal levels of the MAPK phosphatases MKP-1 and MKP-3, therefore inhibiting the neural peripheral flogosis mediated by MAPK. Accordingly, dietary cocoa was reported to repress the expression of the inducible nitric oxide synthase (iNOS) and repress the basal neuronal expression of CGRP.
As is known, iNOS is able to, when activated, favor an increased production of NO, a fundamental free radical gas with effects in starting and magnifying the inflammatory process and pain [8]. It is probable that cocoa repression of CGRP and iNOS expression involves upregulation of MKP and inhibition of MAPK pathways, since both CGRP and iNOS gene expression has been shown to be regulated through multiple pathways, including the MAPK pathways [8].
Moreover, different compounds from cocoa could be able to provide anti-nociceptive effects by affecting neurons and glial cells [9, 10]. Supporting this hypothesis, flavonoids, by repressing the nuclear factor k-light-chain-enhancer of activated B cells (NF-kB) and MAP kinase pathways, seem to be able to decrease the activation of the microglia and inhibit cytokines release [9, 10]. Flavonoids are also reported to have a neuroprotective effect and to preserve cognitive functions by acting against oxidative stress and neuroinflammation [11].
Potential Pain-Modifying Effects
Polyphenols
Evidence from folk medicine suggests that flavonoids derived from medicinal plants may have analgesic effects [1]. Evidence supports that flavonoid glycosides have a wide variety of biological activities, including anti-inflammatory and analgesic activities, with few side effects [12, 13].
The most represented polyphenols in cocoa beans are proanthocyanidins (about 58% of the polyphenol content), catechins (about 37%), and anthocyanidins (about 4%) [14]. Quercetin, clovamide, deoxyclovamide, and trans-resveratrol are also contained in cocoa beans [15].
There is abundant evidence that certain pro-inflammatory cytokines, such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor α (TNF-α), play a specific role in the process of pathological pain [16] and in both in vitro and in vivo studies have reported cocoa’s ability to reduce cytokines, chemokines, reactive oxygen species (ROS), NO, and other molecules involved in inflammatory response [17].
Cocoa extracts or isolated flavonoids have shown anti-inflammatory properties in some in vitro studies. A cocoa extract and some flavonoids (epicatechin and isoquercitrin) reduced macrophage secretion of TNF-α and monocyte chemoattractant protein (MCP)-1. [18]. Similarly, epicatechin suppressed the production of IL-6 and IL-8 in stimulated whole blood cells culture [19].
However, other in vitro studies have shown that some oligomers were able to increase the production of TNF-α, IL-1, and IL-6 in peripheral blood mononuclear cells under stimulation with lipopolysaccharide [20, 21].
Aside from cytokines, cocoa can influence other inflammatory molecules. NO production was reduced by a cocoa extract, epicatechin, and procyanidin B1 and B2 in stimulated macrophages [22, 23]. Furthermore, cocoa and flavonoids were found to reduce the generation of ROS in various types of cells in vitro [24]. In addition to this, in intestinal epithelial cells, hexameric cocoa procyanidins have been reported to modulate the activation of the transcription factor NF-kB, mediated by TNF-α. NF-kB regulates the expression of genes encoding for molecules and enzymes involved in the inflammatory process [cytokines, i-NOS, cyclooxygenase 2 (COX-2), adhesion molecules, acute phase proteins and others] [26].
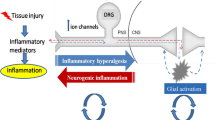
Quercetin has been demonstrated to exert anti-inflammatory, antioxidant, and analgesic actions [27]. A wide spectrum of animal models indicated that quercetin could increase the pain threshold. Various mechanisms seem to be responsible for the analgesic effects of quercetin, involving both the central and the peripheral nervous system. These mechanisms include NO production, activation of γ-aminobutyric acid (GABA) and serotonin receptors, opioid-like effects, and inhibition of transient receptor potential cation channel subfamily V member 1 (TRPV-1)/NMDA receptors, cytokine production, and oxidative stress [27, 28] (Fig. 1).
Cocoa for pain relief. The figure resumes all the putative mechanisms involved in the anti-inflammatory and anti-nociceptive effects of cocoa by compounds: polyphenols [23,24,25,26,27,28,29], methylxanthines [32, 33], N-acylethanolamines [34,35,36], biogenic amines [36,37,38], alkaloids [39,40,41,42], and tryptophan [43,44,45,46,47,48]
Clovamide(N-caffeoyl-3-O-hydroxytyrosine), a typical example of the class of the N-phenylpropenoyl-l-amino acids, is structurally similar to rosmarinic acid, a phenolic compound with anti-inflammatory properties (inhibition of: oxidative burst, cytokine secretion, and NF-kB activation) [3, 29].
These findings suggest that the pain-relieving action of cocoa may be predominantly due to the anti-inflammatory effect of its polyphenols. These compounds may also act through the modulation of opioidergic mechanisms and by interfering with GABAergic systems and NO pathways (Fig. 1).
Methylxanthines
The most important methylxanthines represented in cocoa are caffeine and theobromine [30]. According to this, different analgesic drugs, particularly those containing acetaminophen and aspirin, contain caffeine [31].
In preclinical studies, caffeine has been shown to exhibit several effects on nociception, depending on dose, nociceptive test, stimulus intensity, and species. The action of some analgesics (acetaminophen, amitriptyline, oxcarbazepine, cizolirtine) is inhibited by low doses of caffeine. The underlying mechanism might be the block of central adenosine A1 receptors [31]. At higher doses (approximately 15–45 mg/kg), caffeine augments antinociception by acetaminophen and others non-steroidal anti-inflammatory drugs (NSAIDs) and produces intrinsic antinociception in some preclinical models. At even higher doses (50–100 mg/kg), it shows an intrinsic antinociceptive action in a wide range of tests. The systems involved in the antinociceptive action include the role of the central cholinergic and noradrenergic pathways as well as the blockade of the central adenosine receptors A2B and A2A, and of the microglial COX [32].
Various evidence in this sense suggests how paracetamol and NSAIDs with added caffeine can act with an adjuvant effect on intrinsic analgesia in different headache conditions. In this regard, antinociceptive action within the headache is thought to involve modulation of central blood flow [32].
Mechanisms implicated in anti-nociceptive and adjuvant effects involve inhibition of central adenosine A2B (and possibly A2A) receptors, inhibition of COX in microglia, engagement of central cholinergic systems, and involvement of central noradrenergic systems [32].
Clinical studies with caffeine have indicated adjuvant analgesic actions in combination with acetaminophen and NSAIDs and intrinsic analgesia in several headache conditions. Efficacy in headache states may involve alterations in central blood flow [32].
All of these findings suggest that cocoa might act as a modulator of painful sensations also by virtue of its methylxanthine content (Fig. 1).
Anandamide
In almost all the steps of the pain pathway, cannabinoid receptors and ligands have been described as having a pivotal role [33].
The two most abundant N-acylethanolamines found in chocolate (N-oleoylethanolamine and N-linoleylethanolamine) are reported not to activate brain cannabinoid receptors, but to inhibit anandamide degradation, thus causing the accumulation of non-metabolized anandamide at its sites of action [34]. Anandamide, an endogenous cannabinoid, is only present in small concentrations and, in addition, is unstable [35]. The efficacy in vivo of these compounds needs further investigations [35].
This highlights that the opioid pathway is also one of the possible mechanisms by which cocoa may exhibit its analgesic action (Fig. 1).
Biogenic Amines
The psychoactive constituents of cocoa include biogenic amines, such as tyramine and phenylethylamine (PEA), which are only present in low concentrations [36].
A large amount of evidence indicates that a possible neuromodulator of the aminergic synapses may be represented by the PEA. With regard to this, in fact, in the event of depression, it has been observed that the main metabolite of PEA, represented by phenylacetic acid, is reduced in the biological fluids of patients affected by this pathological condition [37].
A possible diatribe also sees the relationship between cocoa and PEA as a possible causal condition of migraine. Brain PEA may be a neuromodulator of aminergic synapses. Phenylacetic acid, the main metabolite of PEA, is decreased in the biological fluids of depressed subjects [37].
One interesting controversy around chocolate and PEA is the question of whether chocolate can cause migraines and whether this may be due to PEA. In fact, there are other foods containing PEA, tyramine, or histamine (for example cheese and wine), which are also supposed to trigger migraines. However, results among various studies are inconclusive and the relationship between chocolate and migraines still has to be clarified [38] (Fig. 1).
Alkaloids
Cocoa also contains salsolinol, up to a concentration of 25 μg/g. This tetrahydroisoquinoline alkaloid is a dopaminergic active compound [39].
In particular, salsolinolis referred to bind to the dopamine D3-receptor involved in the reward system [3]. Moreover, it seems to be able to activate μ-opioid receptors on GABAergic neurons in rats [40]. However, it has a poor ability to cross the blood–brain barrier [41]. The analgesic action of salsolinol seems to also involve a peripheral mechanism [42] (Fig. 1).
Tryptophan
Chocolate contains serotonin (5-HT) and its precursor, tryptophan (TRP) [43]. Selective serotonin reuptake inhibitors (SSRIs), which increase serotonin levels in the brain, are effective antidepressants. They are currently also used for the management of chronic pain.
Seltzer et al. [44] showed an improvement in pain tolerance and mood after TRP supplementation in healthy subjects. Despite these findings, acute TRP depletion seemed not to affect cold pressure pain in a study by Abbott et al. [45], which also demonstrated that acute TRP depletion was able to inhibit morphine-induced analgesia. These findings suggested a complex role of 5-HT in the modulation of pain [45]. Research by Martin et al. [46] found that acute TRP depletion decreased both heat pain tolerance and threshold. Furthermore, acute TRP depletion was reported to decrease brain 5-HT levels, and to increase visceral perception and negative emotional bias in patients with irritable bowel syndrome [47].
Because of its content in 5-HT and TRP, cocoa might influence the perception of pain, as the neurotransmitter serotonin is involved in the molecular mechanisms underlying pain transmission and mood regulation [48] (Fig. 1).
Future Perspectives in Treatment of Pain
To date, the pharmacological approaches to treat neuropathic pain (NP) are not encouraging. To improve the current knowledge on NP and its underlying mechanisms and to discover new therapeutic molecules, several animal models of NP have been generated [49].
Flavonoids are considered a promising alternative to alleviate NP, especially because of their anti-inflammatory properties [50].
From this point of view, quercetin is an extremely interesting molecule, because it interferes with peculiar biological pathways involved in inflammation process [50]. In fact, quercetin has demonstrated a good ability to reduce the expression of various interleukins (such as IL-6 and IL-2) and also iNOS, NF-κB, p38 MAPK, and TNF-α levels. In addition to this, quercetin has been shown to attenuate heat hypersensitivity and mechanical allodynia and also to reduce pronociceptive cytokine production and the oxidative imbalance of inflammatory pain. Quercetin chronic treatment has been able to ameliorate cold allodynia as well as hyperalgesia. Quercetin has also been evaluated in treating cancer pain, demonstrating a good analgesic activity in Ehrlich tumor-induced hyperalgesia [50].
Epigallocatechin-3-gallate (EGCG), the most important tea catechin, has been reported to be able to relieve pain through its anti-inflammatory and antioxidant activity [49]. EGCG has been described to have antinociceptive actions and to exert beneficial effects on neuronal damage in animal models, with a positive influence on peripheral nerve injuries, thermal hyperalgesia, and diabetic neuropathy. EGCG seems to interfere with different molecular signaling pathways involved in NP. Furthermore, a possible role of EGCG in ameliorating bone cancer pain has been shown [49].
Other polyphenolic compounds have been reported to exert beneficial effects on NP, such as hesperidin [51], diosmin [52], naringenin [53].
Although there is increasing evidence from preclinical studies of analgesic properties of flavonoids, there are not at present clinical trials supporting these results in humans. Therefore, further research is needed [49].
The reported results on cocoa suggest that putative pathophysiological mechanisms specifically involved in pain might be positively affected and modulated by cocoa flavonoids. Starting from this point of view, further research should be addressed to this topic aiming to support clinical evidence on the peculiar anti-nociceptive effects of cocoa.
Conclusions
Cocoa is a flavonoid-rich food with worldwide popularity. Results from epidemiological, preclinical, and human intervention studies suggest that cocoa may exert positive effects in various chronic pathologies, such as cardiovascular diseases and other conditions related to inflammation and oxidative stress (Fig. 1).
Pain is a global public health problem and constitutes one of the most significant societal issues in both cost and suffering.
Many plants containing polyphenols have been commonly used in popular medicine to relieve pain. Flavonoids have been demonstrated to have antinociceptive properties and they might be useful for the development of new natural analgesics.
The two studies by Cady et al. [7, 8] mentioned above are extremely interesting because they demonstrate a possible action of dietary cocoa in relieving pain, and provide novel mechanistic data about the effects of cocoa in relevant animal models.
However, further research is needed to better identify the active principles in cocoa, to understand the underlying mechanisms of action, and to establish efficacy in humans.
References
Xiao X, Wang X, Gui X, Chen L, Huang B. Natural flavonoids as promising analgesic candidates: a systematic review. ChemBiodivers. 2016;13(11):1427–40.
Andújar I, Recio MC, Giner RM, Ríos JL. Cocoa polyphenols and their potential benefits for human health. Oxid Med Cell Longev. 2012;2012:906252.
Tuenter E, Foubert K, Pieters L. Mood components in cocoa and chocolate: the mood pyramid. PlantaMed. 2018;84(12–13):839–44.
Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15(10):2779–811.
Abbey MJ, Patil VV, Vause CV, Durham PL. Repression of calcitonin gene-related peptide expression in trigeminal neurons by a Theobroma cacao extract. J Ethnopharmacol. 2008;115(2):238–48.
Bowden LN, Rohrs EL, Omoto K, Durham PL, Holliday LS, Morris AD, Allen KD, Caudle RM, Neubert JK. Effects of cocoa-enriched diet on orofacial pain in a murine model. OrthodCraniofac Res. 2017;20(Suppl 1):157–61.
Cady RJ, Denson JE, Durham PL. Inclusion of cocoa as a dietary supplement represses expression of inflammatory proteins in spinal trigeminal nucleus in response to chronic trigeminal nerve stimulation. Mol Nutr Food Res. 2013;57(6):996–1006.
Cady RJ, Durham PL. Cocoa-enriched diets enhance expression of phosphatases and decrease enriched diets enhance expression of phosphatases and decrease expression of inflammatory molecules in trigeminal ganglion neurons. Brain Res. 2010;1323:18–32.
Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33(1):83–97.
Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr Pharm Des. 2009;15(10):1072–84.
Grassi D, Ferri C, Desideri G. Brain protection and cognitive function: cocoa flavonoids as nutraceuticals. Curr PharmDes. 2016;22(2):145–51.
Shah AS, Alagawadi KR. Anti-inflammatory, analgesic and antipyretic properties of Thespesia populnea Soland ex. Correa seed extracts and its fractions in animal models. J Ethnopharmacol. 2011;137(3):1504–9.
Shukla S, Mehta A, Mehta P, Vyas SP, Shukla S, Bajpai VK. Studies on anti-inflammatory, antipyretic and analgesic properties of Caesalpinia bonducella F. seed oil in experimental animal models. Food ChemToxicol. 2010;48(1):61–4.
Khan N, Khymenets O, Urpí-Sardà M, Tulipani S, Garcia-Aloy M, Monagas M, Mora-Cubillos X, Llorach R, Andres-Lacueva C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients. 2014;6(2):844–80.
Jalil AM, Ismail A. Polyphenols in cocoa and cocoa products: is there a link between antioxidant properties and health? Molecules. 2008;13(9):2190–219.
Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37.
Pérez-Cano FJ, Massot-Cladera M, Franch A, Castellote C, Castell M. The effects of cocoa on the immune system. Front Pharmacol. 2013;4:71.
Ramiro E, Franch A, Castellote C, Pérez-Cano F, Permanyer J, Izquierdo-Pulido M, et al. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J Agric Food Chem. 2005;53:8506–11.
Al-Hanbali M, Ali D, Bustami M, Abdel-Malek S, Al-Hanbali R, Alhussainy T, Qadan F, et al. Epicatechin suppresses IL-6, IL-8 and enhances IL-10 production with NF-κB nuclear translocation in whole blood stimulated system. Neuro Endocrinol Lett. 2009;30:131–8.
Mao TK, Van de Water J, Keen CL, Schmitz HH, Gershwin ME. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J Med Food. 2002;5:17–22.
Kenny TP, Keen CL, Schmitz HH, Gershwin ME. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp Biol Med. 2007;232:293–300.
Ono K, Takahashi T, Kamei M, Mato T, Hashizume S, Kamiya S, et al. Effects of an aqueous extract of cocoa on nitric oxide production of macrophages activated by lipopolysaccharide and interferon-γ. Nutrition. 2003;19:681–5.
Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673–83.
Ramiro-Puig E, Casadesús G, Lee HG, Zhu X, McShea A, Perry G, et al. Neuroprotective effect of cocoa flavonoids on in vitro oxidative stress. Eur J Nutr. 2009;48:54–61.
Erlejman AG, Jaggers G, Frag CG, Oteiza PI. TNFα-induced NF-kB activation and cell oxidant production are modulated by hexameric procyanidins in Caco-2 cells. Arch Biochem Biophys. 2008;476:186–95.
Pahl HL. Activators and target genes of Rel/NF-kB transcription factors. Oncogene. 1999;18:6853–66.
Borghi SM, Pinho-Ribeiro FA, Fattori V, Bussmann AJ, Vignoli JA, Camilios-Neto D, Casagrande R, Verri WA Jr. Quercetin inhibits peripheral and spinal cord nociceptive mechanisms to reduce intense acute swimming-induced muscle pain in mice. PLoS One. 2016;11(9):e0162267.
Calixto-Campos C, Corrêa MP, Carvalho TT, Zarpelon AC, Hohmann MS, Rossaneis AC, Coelho-Silva L, Pavanelli WR, Pinge-Filho P, Crespigio J, Bernardy CC, Casagrande R, Verri WA Jr. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal Cell Pathol (Amst). 2015;2015:285708.
Zeng H, Locatelli M, Bardelli C, Amoruso A, Coisson JD, Travaglia F, Arlorio M, Brunelleschi S. Anti-inflammatory properties of clovamide and Theobroma cacao phenolic extracts in human monocytes: evaluation of respiratory burst, cytokine release, NF-κB activation, and PPARγ modulation. J Agric Food Chem. 2011;59(10):5342–50.
Franco R, Oñatibia-Astibia A, Martínez-Pinilla E. Health benefits of methylxanthines in cacao and chocolate. Nutrients. 2013;5(10):4159–73.
Sawynok J. Methylxanthines and pain. Handb Exp Pharmacol. 2011;200:311–29.
Sawynok J. Methylxanthines. In: Fredholm BB, editor. Handbook of experimental pharmacology 200. Berlin: Springer; 2011. p. 311–29.
Burston JJ, Woodhams SG. Endocannabinoid system and pain: an introduction. Proc Nutr Soc. 2014;73(1):106–17.
Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E, Mechoulam R. Trick or treat from food endocannabinoids? Nature. 1998;396(6712):636–7.
Di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382(6593):677–8.
Parker G, Parker I, Brotchie H. Mood state effects of chocolate. J Affect Disord. 2006;92(2–3):149–59.
Sabelli HC, Javaid JI. Phenylethylamine modulation of affect: therapeutic and diagnostic implications. J Neuropsychiatry Clin Neurosci. 1995;7(1):6–14.
Irsfeld M, Spadafore M, Prüß BM. β-phenylethylamine, a small molecule with a large impact. Webmedcentral. 2013;4(9):4409.
Melzig MF, Putscher I, Henklein P, Haber H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J Ethnopharmacol. 2000;73(1–2):153–9.
Xie G, Hipólito L, Zuo W, Polache A, Granero L, Krnjevic K, Ye JH. Salsolinol stimulates dopamine neurons in slices of posterior ventral tegmental area indirectly by activating μ-opioid receptors. J Pharmacol Exp Ther. 2012;341(1):43–50.
Melis M, Carboni E, Caboni P, Acquas E. Key role of salsolinol in ethanol actions on dopamine neuronal activity of the posterior ventral tegmental area. Addict Biol. 2015;20(1):182–93.
Eschalier A, Durif F, Fialip J, Aumaitre O, Marty H, Dordain G. Antinociceptive activity of salsolinol (racemate, R(+)-, S(−)-enantiomers): evidence of a peripheral mechanism. Life Sci. 1989;45(5):431–8.
Guillén-Casla V, Rosales-Conrado N, León-González ME, Pérez-Arribas LV, Polo-Díez LM. Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection. J Chromatogr A. 2012;1232:158–65.
Seltzer S, Stoch R, Marcus R, Jackson E. Alteration of human pain thresholds by nutritional manipulation and l-tryptophan supplementation. Pain. 1982;13(4):385–93.
Abbott FV, Etienne P, Franklin KB, Morgan MJ, Sewitch MJ, Young SN. Acute tryptophan depletion blocks morphine analgesia in the cold-pressor test in humans. Psychopharmacology. 1992;108(1–2):60–6.
Martin SL, Power A, Boyle Y, Anderson IM, Silverdale MA, Jones AKP. 5-HT modulation of pain perception in humans. Psychopharmacology (Berl). 2017;234(19):2929–39.
Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TO, Evers EA, Backes WH, Brummer RJ, van Nieuwenhoven MA. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60(9):1196–203.
Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015;12(1):1–8.
Bimonte S, Cascella M, Schiavone V, Mehrabi-Kermani F, Cuomo A. The roles of epigallocatechin-3-gallate in the treatment of neuropathic pain: an update on preclinical in vivo studies and future perspectives. Drug Des Dev Ther. 2017;11:2737–42.
Carullo G, Cappello AR, Frattaruolo L, Badolato M, Armentano B, Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med Chem. 2017;9(1):79–93.
Carballo-Villalobos AI, González-Trujano ME, Alvarado-Vázquez N, López-Muñoz FJ. Pro-inflammatory cytokines involvement in the hesperidin antihyperalgesic effects at peripheral and central levels in a neuropathic pain model. Inflammopharmacology. 2017;25(2):265–9.
Bertozzi MM, Rossaneis AC, Fattori V, Longhi-Balbinot DT, Freitas A, Cunha FQ, Alves-Filho JC, Cunha TM, Casagrande R, Verri WA Jr. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem Biol Interact. 2017;273:180–9.
Manchope MF, Casagrande R, Verri WA Jr. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 2017;8(3):3766–7.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Martina De Feo, Antonella Paladini, Claudio Ferri, Augusto Carducci, Rita Del Pinto and Davide Grassi have nothing to disclose. Giustino Varrassi is an Editor-in-Chief of this journal.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12024564.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
De Feo, M., Paladini, A., Ferri, C. et al. Anti-Inflammatory and Anti-Nociceptive Effects of Cocoa: A Review on Future Perspectives in Treatment of Pain. Pain Ther 9, 231–240 (2020). https://doi.org/10.1007/s40122-020-00165-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-020-00165-5