Abstract
Introduction
Antimicrobial resistance (AMR) is one of the most serious public health challenges worldwide, including in Japan. However, there is limited evidence assessing the AMR burden in Japan. Thus, this systematic literature review (SLR) and meta-analysis (MA) were conducted to assess the clinical and economic burden of AMR in Japan.
Methods
Comprehensive literature searches were performed on EMBASE, MEDLINE, the Cochrane Library, and ICHUSHI between 2012 and 2022 following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. MA estimated a pooled effect between the two comparative arms (AMR vs. non-AMR). The results were reported in measures of odds ratios (ORs) for in-hospital mortality and in standardized mean differences (SMDs) for length of stay (LOS) and direct medical costs.
Results
Literature searches identified 1256 de-duplicated records, of which 56 observational studies (English, n = 35; Japanese, n = 21) were included. Of note, twenty-two studies (39.3%) compared the AMR group with non-AMR group. In the SLR, in-hospital mortality, LOS, and direct medical costs were higher in the AMR group compared to the non-AMR group. Eight studies were selected for the MA. In the AMR group, the pooled estimate showed a statistically higher in-hospital mortality [random effect (RE)—OR 2.25, 95% CI 1.34–3.79; I2 = 89%; τ2 = 0.2257, p < 0.01], LOS (RE—SMD 0.37, 95% CI − 0.09–0.84; I2 = 99%; τ2 = 0.3600, p < 0.01), and direct medical cost (RE—SMD 0.53, 95% CI 0.43–0.62; I2 = 0.0%; τ2 = 0.0, p = 0.88) versus the non-AMR group.
Conclusion
Our study presents an overview of the clinical and economic burden of AMR in Japan. Patients with AMR infections experience significantly higher in-hospital mortality, LOS, and direct medical costs compared with patients without AMR infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Bacterial antimicrobial resistance (AMR) has emerged as one of the leading public health threats of the twenty-first century, with 1.27 million deaths in 2019 attributed to it globally. |
Several systematic literature reviews and meta-analyses have been conducted on the disease burden of AMR globally; however, there is limited evidence assessing the AMR burden in Japan. |
What was learned from the study? |
Methicillin-resistant Staphylococcus aureus (MRSA) is the most researched and published AMR infection type in Japan. |
AMR is associated with a high level of in-hospital mortality, length of stay and direct medical costs among the Japanese population. |
There is country-wise diversity among the antibiotic-resistant population, and few studies have assessed the comprehensive burden between AMR and non-AMR groups. |
Extensive research on the topic is needed to better understand the overall burden of AMR in Japan. |
Introduction
Antimicrobials are medications used to prevent and treat infections. These include antibiotics, antivirals, antifungals, and antiparasitic drugs. Antimicrobial resistance (AMR) occurs when pathogens develop mechanisms to thrive or survive in the presence of the drug. As a result, antimicrobials show reduced clinical efficacy, and infections become increasingly difficult or impossible to treat. These result in an increased risk of disease spread, severe illness, and death [1]. The irrational use of antibiotics is directly correlated with the increase in AMR and varies from country to country, depending on the respective antibiotic usage patterns [2, 3].
The global review on AMR estimated 700,000 deaths in 2014 attributable to drug-resistant strains of common bacterial infections, human immunodeficiency virus (HIV), tuberculosis, and malaria [4]. However, bacterial AMR emerged as one of the leading public health threats of the twenty-first century, with 1.27 million deaths directly caused by bacterial AMR globally in 2019 [5]. The total annual number of deaths caused by AMR in Japan is unknown; however, a 2020 study by Tsuzuki et al. estimated that, in 2017, the number of deaths attributed to bloodstream infections (BSIs) caused by methicillin-resistant Staphylococcus aureus (MRSA) and fluoroquinolone-resistant Escherichia coli (FQREC) was approximately 8100 [6]. According to the Japan Nosocomial Infections Surveillance (JANIS) Annual Open Report 2022, the resistance rates of third-generation cephalosporin antibiotics, including cefotaxime and ceftazidime, were 26.8% and 12.8% for E. coli and 12.6% and 10.3% for Klebsiella pneumoniae, respectively, whereas for levofloxacin, the resistance rate to E. coli was 39.6% [7]. An analysis of 2289 hospitals across Japan, conducted as part of the JANIS program 2022, reported that the percentage of hospitals reporting at least one corresponding specific AMR bacteria in 2022 was the highest for MRSA (99.7%), followed by penicillin-resistant Streptococcus pneumoniae (PRSP; 53.9%) and carbapenem-resistant Enterobacteriaceae (CRE; 51.2%) [7].
Rising AMR has severe health and economic consequences [8]. A 2018 report by the Organization for Economic Co-operation and Development (OECD) that analyzed 33 OECD, European Union (EU), and European Economic Area countries estimated that AMR caused a total of $3.5 billion annually in health care costs. In Japan, Matsumoto et al. [9] estimated the clinical and economic outcomes of drug-resistant gram-negative pathogens in Japan and demonstrated that economic and clinical values could be considerably increased by reducing AMR levels in the country. It was reported that savings of ¥2.5 billion ($23 million) to ¥6.4 billion ($60 million) in hospitalization costs could be achieved in a year by reducing AMR by 50% in Japan [9].
In March 2021, the Japan Agency for Medical Research and Development (AMED) Public and Private Partnerships for Infectious Diseases R&D created the first edition of the Japanese version of the Priority Pathogen List, which was approved by seven academic societies and the Drug Discovery Promotion Review Committee [10].
In Japan, there is limited evidence assessing AMR burden. Since AMR strains and resistance rates that require attention differ from country to country, there is a need to appropriately assess the country-wise burden of AMR by referring to previous data and reports. This study aims to conduct a systematic literature review (SLR) and meta-analysis (MA) to determine the clinical and economic burden of AMR in Japan.
Methods
Overview
The searches were designed by considering the combination of sensitivity and specificity. The SLR was conducted in accordance with the general recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [11], the general principles of the Centre for Reviews and Dissemination (University of York) guidance [12] for undertaking reviews in health care, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13].
Eligibility Criteria
Table 1 contains the eligibility criteria defined under the Population, Intervention, Comparison, Outcomes, and Study design (PICOS) framework [14]. The systematic literature search was conducted using a predefined search strategy to identify eligible studies; the search strategies are presented in Supplementary Table 1. The target population for this SLR was adult Japanese patients (≥ 18 years of age) with at least one AMR infectious disease for any treatment. The focus was on interventional and observational studies assessing the clinical burden [in-hospital mortality, and length of stay (LOS)—including intensive care unit (ICU) and isolation bed stays] and economic burden (direct medical costs, duration of antibiotics, and amount of antibiotic usage) of AMR in Japan.
The definition of AMR in this study refers to the first edition of the Japanese version of the Priority Pathogen List that was created by the AMED Public and Private Partnerships for Infectious Diseases R&D and which was approved by seven academic societies and the Drug Discovery Promotion Review Committee [10].
Data Sources
Evidence published between 2012 and 2022 was sourced from various databases, including EMBASE, MEDLINE, the Cochrane Library (via Ovid SP®), and ICHUSHI (a Japanese database). Gray literature and conference abstracts for the last 3 years were also hand-searched to identify records on the treatments used for AMR infection in Japan in the following databases: Infectious Diseases Society of America, European Congress of Clinical Microbiology & Infectious Diseases/European Society of Clinical Microbiology and Infectious Diseases, International Society for Pharmacoeconomics and Outcomes Research, The Japanese Association for Infectious Diseases, Japanese Society of Chemotherapy, and Japan Society of Environmental Infectious.
Search Strategy
The search strategy was developed by combining free-text words, indexing terms, and Boolean terms with terms pertinent to disease areas, interventions, and study designs. The search strings were modified to conform to the syntax of each database. According to the eligibility criteria, all retrieved studies were then evaluated. Two reviewers independently assessed the eligibility (inclusion/exclusion) of titles and abstracts identified during the search using predetermined criteria using the PICOS framework. Every instance of uncertainty or ambiguity was resolved by consulting a third independent reviewer. Publications selected as potentially relevant from the abstract screening were retained for the full-text review. Two independent reviewers evaluated full-text publications, and discrepancies were resolved by consulting a third independent reviewer.
Data Extraction
A standardized MS Excel® data extraction template was used to conduct data extraction from the full-text studies identified by the searches. The key methodology, patient characteristics, and results were extracted and tabulated for each study. One researcher performed data extraction, which was checked by another independent researcher. The results of the data extraction were used for the feasibility assessment and the MA. Means, medians, standard deviations (SDs), 95% confidence intervals (95% CIs), and interquartile ranges (reporting 25th and 75th values) were used to report continuous outcomes, while counts and percentages were used to report categorical outcomes. For the comparison of direct medical costs in the MA, the selected cost data of the studies were adjusted to represent the values for 2022 in local currency [15]. The annual exchange rate for 2022 by the OECD was used to convert Japanese yen to United States (US) dollars ($1 = ¥131.498) [16]. Other cost data were presented as reported in the studies.
Quality Assessment
The quality of randomized controlled trial (RCT) studies included in the SLR was evaluated to ensure that this review's conclusions and findings are based on the best available evidence and to identify any potential sources of bias in the data. The quality of RCT studies retained for data extraction was assessed using a Cochrane risk of bias tool for randomized trials. This checklist (also called “RoB”) is the most recommended tool for RCTs. The RoB 2.0 tool is suitable for individually randomized, parallel-group, and cluster-randomized trials [17]. The Newcastle-Ottawa scales for cohort case-control studies were used to evaluate the risk of bias in each individual article included in this study [18] (Supplementary Table 2). Gray literature that did not encompass full-text articles was evaluated using the Authority, Accuracy, Coverage, Objectivity, Date, Significance checklist [19].
Data Analysis
Statistical Analysis
The MA estimated the clinical and economic burden between the two comparative arms: the AMR (resistant) and non-AMR (susceptible) arms. A high degree of heterogeneity was estimated because of differences in underlying diseases, causative organisms, patient backgrounds, and clinical characteristics among individual studies. The MA was performed only on studies when they used design and/or analysis for adjustment of confounders, such as propensity score matching performed on the two comparative arms. All analyses were performed using the R software environment for statistical computing and graphics (version 4.1 or above). The ‘meta,’ ‘metafor,’ package in R was used to conduct the analyses.
The MA was used to estimate the pooled effect between the two arms (i.e., summary of proportions, time-to-event outcomes, and mean of outcomes) across all eligible studies. The weighted average of each outcome measure of the studies was calculated using inverse variance weighting to estimate the pooled effect [20]. Both random effect (RE) and fixed effect (FE) models were applied; however, the RE model was selected as the primary model [20]. The results were reported with the measure of effect as odds ratios (ORs) and standardized mean differences (SMDs) with 95% CIs. In-hospital mortality was reported with dichotomous outcomes. Also, LOS and direct medical costs were reported as continuous outcomes. The Box-Cox method [21] was applied to studies not reporting mean and SD directly to estimate the mean and SD according to the reported sample size, median, minimum value, maximum value, first quartile, or third quartile.
Assessment of Statistical Heterogeneity and Publication Bias
Heterogeneity among the selected studies was evaluated using both the Q test and I2 index [22]. In the Q test, a p value cutoff < 0.1 for the test for heterogeneity indicated presence of heterogeneity. The I2 index (0–40% no heterogeneity, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity, and 75–100% considerable heterogeneity) was used to describe the percentage of total variation across analyses due to heterogeneity [11, 23]. In case of existing substantial heterogeneity in MA and sufficient studies, subgroup analysis was conducted according to the characteristic of the study or patient, including studies in MA. Moreover, as another analysis, the study with extreme value, which might lead to substantial heterogeneity, was excluded in MA.
Publication bias was assessed through funnel plot for base case analysis in this study, Egger’s test was not applicable because of the small number of studies for each outcome (n < 10 studies) [24, 25].
Ethical Approval
Ethics approval was not required for this study. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Overview
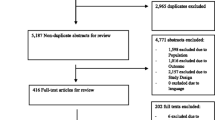
A search of the databases returned a total of 1262 results. Following the removal of duplicates and a thorough review, 57 records (consisting of 56 studies) that met the inclusion criteria were included in the SLR (Supplementary Table 3). Figure 1 presents the PRISMA flow diagram for the SLR, and Supplementary Table 4 presents the PRISMA check list.
Study Characteristics
For the SLR, a total of 56 studies (35 in English and 21 in Japanese) were included. The details of these studies are presented in Supplementary Table 5. All studies were conducted in Japan, with the majority being conducted in single-center settings (62.5%), followed by multicenter settings (33.9%) and unclear settings (3.6%). Fifty-three of the 56 studies (94.6%) were cohort studies, two (3.6%) were case-control studies, and one (1.8%) was a cross-sectional study. Twenty-two studies (39.3%) compared the AMR group with the non-AMR group. The overall sample size ranged from 33 to 7,772,050 participants, while the sample size for resistant populations ranged from 12 to 93,838 participants. Fourteen of the 56 studies (25.0%) included patients with BSI, nine (16.1%) included patients with pneumonia, four (7.1%) included patients with surgical site infection, and two studies each (3.6%) included patients with urinary tract infection and sepsis. One study each (1.8%) enrolled patients infected with vertebral osteomyelitis and invasive pneumococcal disease, and the remaining twenty-three studies (41.1%) included patients with multiple infections. Twenty-nine (51.8%) of the 56 studies were on MRSA, and S. aureus was the most common causative pathogen (53.6%). Table 2 presents details of the study characteristics.
Baseline Patient Characteristics
Supplementary Table 6 presents the baseline characteristics of the patients in the 56 included studies. The median age distribution of the population ranged from 60 to 89 years. The average body mass index (BMI) varied between 20.8 and 22.9 kg/m2. There were marginally more male than female participants. The majority of the population in this study included patients with MRSA infections. Average Sequential Organ Failure Assessment (SOFA) scores ranged between 4.7 and 8.5. A total of 51.8% of the patients had a SOFA score ≥ 2, indicating that the infection was associated with an increased risk of death or prolonged hospital stay. The median Charlson Comorbidity Index (CCI) score ranged from 0.99 to 3.9. The proportion of patients with CCI scores ≥ 3 and ≥ 2 was greater in the resistant group (69.0% and 45.4%, respectively) than in the susceptible group (35.0% and 35.9%, respectively). The most prevalent comorbidity was diabetes.
Clinical Outcomes
In-Hospital Mortality
Forty studies reported in-hospital mortality (which includes 7-, 28-, 30-, and 90-day mortality) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. See Supplementary Table 7 for more details and other results of the SLR.
Twenty-four studies evaluated MRSA [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65], and of those, seventeen studies reported in-hospital mortality, which ranged from 0% [54, 59] to 73.0% [44]. The MRSA group had significantly higher in-hospital mortality compared with the non-MRSA group (31.2% vs. 11.6%, p < 0.001) [48] and the methicillin-susceptible S. aureus (MSSA) group (36.7% vs. 15.0%, p = 0.012) [62]. In 2016, Uematsu et al. compared in-hospital mortality between an anti-MRSA group and a control group and found that the anti-MRSA group had significantly higher in-hospital mortality (22.6% vs. 6.2%, p < 0.001, respectively) than the control group [56].
Three studies [39,40,41] evaluated carbapenem-resistant patients, and the in-hospital mortality rate ranged from 8.1 to 36.4% [40]. In a 2022 study by Imai et al., the carbapenem-resistant group had higher in-hospital mortality than the susceptible group; however, the difference was not statistically significant (25.6% vs. 21.9%, p = 0.407, respectively) [41].
A significant association among lower BMI, lower Barthel Index, higher Hugh-Jones grade, higher A-DROP score, C-reactive protein ≥ 20 mg/dl or infiltration of at least two-thirds of one lung, mechanical ventilation at admission, interstitial lung disease, aspiration pneumonia, a high CCI score, a high SOFA score, and having a vancomycin minimum inhibitory concentration ≥ 1.5 µg/ml was observed with increased in-hospital mortality in the MRSA group. Meanwhile, in carbapenem-resistant patients, older age, male gender, immunosuppressive drug use, pneumonia, sepsis, and a CCI score ≥ 1 were significantly associated with increased in-hospital mortality.
Length of Stay
A total of thirty studies reported LOS [26, 27, 31, 40,41,42,43, 47, 49, 52, 54,55,56, 58, 62, 63, 65,66,67,68,69,70,71,72,73,74,75,76,77,78], the details and results of which are presented in Supplementary Table 8. Sixteen studies investigated MRSA infections [42, 43, 47, 49, 52, 54,55,56, 58, 62, 63, 65, 66, 68, 69, 76], for which the mean LOS ranged from 8.6 [54] to 123.6 days [68]. The MRSA population had a significantly longer LOS than the non-MRSA group (35.0 days vs. 14.0 days, p < 0.001, respectively) [63].
Carbapenem-resistant groups [40, 41, 67, 72, 74, 77] were reported in six studies, and the median LOS ranged from 14.0 to 83.0 days [40]. Imai et al. demonstrated in 2022 that carbapenem-resistant infections had a significantly longer LOS than carbapenem-susceptible infections (64.0 days vs. 46.0 days, p < 0.001, respectively), and even after adjusting with the inverse probability of treatment weight method, a similar trend was observed (median: 63.0 days vs. 51.0 days, p = 0.004, respectively) [41].
Economic Outcomes
Direct Medical Costs
In total, fifteen studies reported direct medical costs [41, 56, 58, 59, 62, 63, 65, 67,68,69,70,71, 73, 79, 80]. Supplementary Table 9 presents the details and results of the studies. Nine studies evaluated direct medical costs in the MRSA population [56, 58, 59, 62, 63, 65, 68, 69, 80] with a median hospitalization cost ranging from $496 [62] to $29,596 [65]. The median hospitalization cost in the MRSA group was significantly higher than that in the non-MRSA group ($12,156 vs. $4665, p < 0.001, respectively) [63], as well as in the MSSA group ($26,035 vs. $19,823, p = 0.036, and mean: $15,762 vs. $14,152, p < 0.001, both respectively) [58]. Tsuzuki et al., in 2021, also evaluated the 5-year total intervention cost (including drug, transplantation, radiation, surgery, and blood transfusion costs), which was $475,059 in the MRSA group and $344,418 in the MSSA group [62].
Two studies evaluated the costs in the carbapenem-resistant population [41, 67]. The average hospitalization cost per patient in the carbapenem-resistant group was higher than that in the carbapenem-sensitive group ($1648 vs. $532, p < 0.001, respectively) [67]. In the carbapenem-resistant group, the median costs for consultation ($163 vs. $143, p = 0.238, respectively), laboratory tests ($2498 vs. $1845, p = 0.002, respectively), and hospital stay ($14,307 vs. $10,560, p < 0.001, respectively) were higher than those in the carbapenem-sensitive group [41]. In addition, the cost of the intervention, which includes medications and surgeries, was higher in the carbapenem-resistant group than in the carbapenem-sensitive group [41, 67].
Amount of Antibiotic Usage
Only four studies [50, 55, 77, 80] reported the amount of antibiotic usage; hence, very few data were available. Supplementary Table 10 presents the details and results of the studies. According to a study published in 2015 by Suzuki et al., between 2006 and 2008, the mean amount of vancomycin in MRSA patients was high (2,591.6 g and 2,563.5 g per year, respectively). In contrast, the amount decreased between 2007 and 2009 (2348.8 g and 2384.5 g, respectively). In a study by Ogasawara et al. in 2012, 20 of the 33 patients treated with meropenem were resistant to Pseudomonas aeruginosa. The daily dose of meropenem for the resistant group was 1, and for the non-resistant group, it was 1.5 (the unit of measurement was not specified) [77].
Duration of Antibiotics
In total, twelve studies reported the duration of antibiotics [37, 38, 49, 55, 59, 63, 73,74,75, 77, 79, 81]. Supplementary Table 11 presents the details and results of the studies. The median duration of antibiotic use in MRSA patients ranged from 10.0 to 21.5 days [49, 55], whereas for ESBL bacteremia, it ranged from 9.0 to 15.9 days [38, 75]. In those studies, the duration of antibiotic use was reported for the MRSA (median: 24.0 days, p < 0.001) [63], carbapenem-resistant (mean: 14.9 days) [74], and meropenem-resistant (median: 9.0 days, p > 0.05) [77] populations, which required longer antibiotic treatment than the non-MRSA (median: 9.0 days), non-carbapenem-resistant (mean: 11.0 days), and non-meropenem-resistant (median: 7.0 days) populations.
Pooled Clinical Outcomes
A total of eight studies from the SLR were included for the MA, of which four [41, 56, 62, 63], seven [41, 56, 62, 63, 72, 75, 77], and three [41, 56, 62] studies evaluated in-hospital mortality, LOS, and direct medical costs, respectively. The outcomes of the amount and the duration of antibiotics were not evaluated because of a very limited number of eligible studies.
In-Hospital Mortality
Among the 20,387 patients from the four studies [41, 56, 62, 63], the pooled estimate showed a statistically significantly higher mortality in the AMR group compared with the non-AMR group for both the FE (OR 3.19, 95% CI 2.92–3.48) and RE models (OR 2.25, 95% CI 1.34–3.79, I2 = 89%, τ2 = 0.2257, p < 0.01) (Fig. 2). The funnel plot of in-hospital mortality is shown in Supplementary Fig. 1. The plot of in-hospital mortality represents bias as the dots outside of the triangle.
In-Hospital Mortality Sensitivity Analyses
A series of sensitivity analyses were conducted after excluding the carbapenem-resistant group. Analysis conducted after excluding the carbapenem-resistant population retained only the MRSA population [56, 62, 63], and the results suggested higher mortality in the AMR group versus the non-AMR group, with statistically significant ORs for the FE (OR 3.31, 95% CI 3.02–3.61) and RE (OR 2.84, 95% CI 1.95–4.14; I2 = 79%, τ2 = 0.0735, p < 0.01) models (Supplementary Fig. 2).
Additionally, a sensitivity analysis conducted after excluding the study that might include other antimicrobial resistance mechanisms [63], and it showed similar results (Supplementary Figs. 3 and 4). This study was defined as an outlier study based on the influence analysis.
Length of Stay
Among the 20,662 patients from the seven studies [41, 56, 62, 63, 72, 75, 77], the pooled estimate showed a statistically significantly higher LOS in the AMR group versus the non-AMR group for FE (SMD 1.27, 95% CI 1.23–1.31) and a higher LOS in the AMR group versus the non-AMR group for RE (SMD 0.37, 95% CI − 0.09–0.84, I2 = 99%, τ2 = 0.3600, p < 0.01) models (Fig. 3). The funnel plot of length of stay was shown in Supplementary Fig. 5. The plot of length of stay represents bias as the dots outside of the triangle.
Length of Stay Sensitivity Analyses
A series of sensitivity analyses was conducted after excluding (1) MRSA, ESBL, and meropenem populations and (2) carbapenem-resistant, ESBL, and meropenem populations. The results of the sensitivity analysis conducted after excluding the carbapenem-resistant, ESBL, and meropenem populations retained only the MRSA population [56, 62, 63] (Supplementary Fig. 6). The results showed that the AMR group had a significantly longer LOS compared with the non-AMR group (for FE and RE models, respectively, SMD 1.35, 95% CI 1.31–1.39 and SMD 0.81, 95% CI 0.10–1.53, I2 = 99%, τ2 = 0.3905, p < 0.01). The results of the sensitivity analysis conducted after excluding the MRSA, ESBL, and meropenem populations retained only the carbapenem-resistant population [41, 72] (Supplementary Fig. 7). The results showed that the AMR group had a longer LOS compared with the non-AMR group; however, the results were not statistically significant (for FE and RE models, respectively, SMD 0.05, 95% CI − 0.12 to 0.22 and SMD − 0.04, 95% CI − 0.71 to 0.63, I2 = 93%, τ2 = 0.2162, p < 0.01).
Additionally, a sensitivity analysis conducted after excluding the study [63] which might include other antimicrobial resistance mechanisms showed similar results (Supplemental Figs. 8–10). This study was defined as an outlier study based on the influence analysis.
Direct Medical Cost
Among the 4965 patients from the three studies [41, 56, 62], the pooled estimate showed a statistically significant increase in direct medical costs among the AMR groups compared with the non-AMR groups (for both FE and RE models, SMD 0.53, 95% CI 0.43–0.62, I2 = 0.0%, τ2 = 0.0, p = 0.88) (Fig. 4). The funnel plot of direct medical cost is shown in Supplementary Fig. 11. The plot did not show bias with the limitation of small sample size (i.e., study number) included in MA.
Direct Medical Cost Sensitivity Analyses
The sensitivity analysis was conducted after excluding the carbapenem-resistant population retained only the MRSA population [56, 62] (Supplementary Fig. 12). The analysis showed significantly higher direct medical costs for the AMR group (for both FE and RE models, SMD 0.54, 95% CI 0.43–0.65, I2 = 0.0%, τ2 = 0.0, p = 0.71) compared to the non-AMR group.
Discussion
This SLR and MA were conducted to determine the clinical and economic burden of AMR in Japan. In-hospital mortality and LOS were the clinical outcomes analyzed, while direct medical costs and the duration and amount of antibiotics were the economic outcomes.
Most of the studies (53.6%) included in the SLR evaluated patients with S. aureus infections. In a previous global SLR study, S. aureus was the most identified causative bacterium (23.4%) [82]. In Japan, nearly half of the cases were confirmed to be S. aureus [82]. The newly published Japanese National Action Plan on Antimicrobial Resistance (AMR) 2023–2027 reported that, as of 2020, the MRSA rate in Japan (48.1%) was higher compared to the EU (16.7%) and other developed countries [83]. In addition, this study found only two studies targeting vancomycin resistance. The reason for this is thought to be that the prevalence of vancomycin-resistant enterococci (VRE) is extremely low in Japan. In the 2022 report, the proportion of VRE in Enterococcus faecium was 2.6% [7]. On the other hand, the percentage of VRE in Europe and the US was reported to be 16.8% and 82.1%, respectively [84, 85]. Additionally, AMR-related mortality rates have been reported to vary by region, with rates tending to be higher in developing countries [5]. This is thought to be influenced not only by the prevalence of AMR but also by access to medical care and the state of public health. In this way, to understand the burden of disease caused by AMR in a specific country or region and to compare existing research results, it is necessary to consider differences in the prevalence of AMR and other influences.
In Japan, a 2019 domestic survey reported that approximately 8000 people died in 2017 because of MRSA and FQREC, which are two of the most common drug-resistant bacteria [6]. Japan's AMR Countermeasure Action Plan 2016–2020 set a goal of “reducing the methicillin resistance rate of S. aureus to 20% or less in 2020,” but the actual annual rate of decrease was only 2% [86]. On the other hand, the UK has achieved an annual decrease rate of 5%. By thoroughly implementing infection prevention and control, and promoting the appropriate use of antimicrobials, it has been confirmed that Japan aims to reduce the drug resistance rate to < 20%, which is the same level as in other developed countries. Under these circumstances, countermeasures against MRSA are urgently needed in Japan, and this study was considered to be extremely useful for understanding the clinical and economic burden of AMR and MRSA in Japan.
According to our results, the MRSA group had significantly higher in-hospital mortality, LOS, and median or average hospitalization cost relative to the non-MRSA, MSSA, and control groups [51, 56, 58, 62, 63, 65]. Even after adjusting for multiple variables such as age, sex, CCI, Barthel Index, nosocomial infection, BSI, ICU admission, surgery, and submission dates of positive S. aureus cultures, the correlation remained statistically significant [58]. Published results from developed countries were also in agreement with our results, in that the median LOS was longer for the MRSA group than for the MSSA group (9 days vs. 7 days, p = 0.045 from the US [87], respectively, and 22.5 days vs. 14 days from Canada [88], respectively).
The carbapenem-resistant population displayed a higher in-hospital mortality rate than the non-resistant population; however, the results were not statistically significant because the resistant group had a limited sample size [41], but LOS in the carbapenem-resistant population was significantly longer than that in the non-resistant population [58]. The multi-drug resistant (MDR) population also had a significantly longer LOS compared with the non-MDR population [78]. Hospitalization costs were also greater in the carbapenem-, penicillin-, oxacillin-, cephalosporin-, fluroquinolone-, and gentamicin-resistant groups than in the respective non-resistant groups [41, 67, 70, 73]. Similar reviews conducted by Dadgostar et al. in 2019 [89] and Pulingam et al. in 2022 [3] also reported that AMR imposes a significant economic burden.
The results of our MA revealed that in-hospital mortality, hospital stays, and direct medical costs were significantly greater in the AMR group relative to the non-AMR group. Our results were consistent with another MA conducted by Poudel et al. in 2023, which reported that the resistant group had significantly higher mortality (OR 1.844 [95% CI 1.187–2.865]), LOS (mean: 7.4 days [95% CI 3.4–11.4]), and readmission (OR 1.492 [95% CI 1.231–1.807]). The same study also reported that costs attributable to the resistant group were higher in the resistant group compared with the non-resistant group [90]. Similarly, the MAs conducted by Cosgrove et al. in 2003 [91] (OR = 1.93, 95% CI 1.54–2.42, p < 0.001) and Rödenbeck et al. in 2023 [92] (OR 2.29, 95% CI 1.91–2.75) also suggested that the resistant group had significantly higher mortality than the susceptible group. Furthermore, the SLR conducted by Naylor et al. in 2018 [82] demonstrated that the resistant population had higher mortality and greater costs compared with the non-resistant population.
Several SLRs and MAs on the disease burden of AMR have been conducted globally [82, 90, 93, 94]. These studies highlight that the global burden of AMR is substantial from both economic and clinical perspectives [90]. Resistant bacterial infections are associated with significant mortality [93]. There is considerable variability in burden estimates, which can lead to inaccurate intervention evaluations and poor policy or investment decisions [82]. AMR decision-making and policy should be driven by reliable, unbiased estimates of the effect size, which can be achieved through MAs [94].
Substantial heterogeneity between studies was observed in our MA because of the considerable diversity in antibiotic resistance. Pooled estimates were calculated to integrate studies regardless of the type of antibiotic resistance for the AMR and non-AMR comparisons, and the sensitivity analysis was conducted by sub-grouping antibiotic resistance types. While the MA results of in-hospital mortality and LOS in the pooled estimates showed high heterogeneity, a few results from the sensitivity analysis showed low heterogeneity. On the other hand, the heterogeneity in the pooled estimates and the sensitivity analysis of direct medical costs was consistent.
Although there is limited evidence in this literature review, most of the eligible studies used for the MA were published after 2016; therefore, there is a possibility of data overlapping among the multicenter studies involved in the MAs. For studies that adjusted patient background for both resistance and non-resistance groups, there is a possibility that the patients showed resistance to other antibiotics than those targeted in the study. In Japan, during the study period, the treatment environment has not significantly changed because of almost no new antibiotics being available; however, the prevalence of resistant bacteria may have been changing.
Conclusions
In Japan, AMR is associated with a significant clinical and economic burden that impacts both patients and society. There is a pressing need for payers, providers, and policymakers to make informed decisions regarding interventions to mitigate AMR-associated burdens. However, since AMR strains and resistance rates show country-wise diversity, appropriately assessing the burden of AMR by referring to existing data and reports is of utmost importance.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files. Except for confidential data associated with data analysis, all data are available from the corresponding author upon reasonable request.
References
World Health Organization. Antimicrobial resistance. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
Altarac D, Gutch M, Mueller J, Ronsheim M, Tommasi R, Perros M. Challenges and opportunities in the discovery, development, and commercialization of pathogen-targeted antibiotics. Drug Discov Today. 2021;26(9):2084–9.
Pulingam T, Parumasivam T, Gazzali AM, Sulaiman AM, Chee JY, Lakshmanan M, et al. Antimicrobial resistance: prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur J Pharm Sci. 2022;170: 106103.
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations: The Review on Antimicrobial Resistance. 2016. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
Antimicrobial RC. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55.
Tsuzuki S, Matsunaga N, Yahara K, Gu Y, Hayakawa K, Hirabayashi A, et al. National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J Infect Chemother. 2020;26(4):367–71.
Japan Nosocomial Infections Surveillance (JANIS). Annual Open Report 2022. 2023. Available from: https://janis.mhlw.go.jp/english/report/open_report/2022/3/1/ken_Open_Report_Eng_202200_clsi2012.pdf.
Van Katwyk SR, Grimshaw JM, Hoffman SJ. Ten Years of inaction on antimicrobial resistance: an environmental scan of policies in Canada from 2008 to 2018. Healthc Policy. 2020;15(4):48–62.
Matsumoto T, Darlington O, Miller R, Gordon J, McEwan P, Ohashi T, et al. Estimating the economic and clinical value of reducing antimicrobial resistance to three gram-negative pathogens in Japan. J Health Econ Outcomes Res. 2021;8(2):64–75.
AMED Public and Private Partnerships for Infectious Diseases R&D. Priority Pathogens Lists for R&D of New Antibiotics (2021 version). 2021. Available from: https://id3catalyst.jp/apid/en/list.html.
The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. 2023. Available from: https://training.cochrane.org/handbook/current.
Centre for Reviews and Dissemination, University of York. Systematic Reviews: CRD's guidance for undertaking reviews in health care. 2009. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37(6):387.
Medical Data Vision. Part #1: 2022 Reform of Medical Fee Structure; what you see, what you don’t. 2022. Available from: https://en.mdv.co.jp/ebm/column/article/13.html.
Organisation for Economic Co-operation and Development. OECD Exchange rates. 2022. Available from: https://data.oecd.org/conversion/exchange-rates.htm.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7.
Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, et al., editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014.
Tyndall J. The AACODS checklist. 2010. Available from: https://www.library.sydney.edu.au/research/systematic-review/downloads/AACODS_Checklist.pdf.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29.
McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. 2020;62(1):69–98.
Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. Boca Raton: Chapman & Hall/CRC Press; 2021. p. 2021.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343: d4002.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Suehiro T, Takahashi Y, Okushita Y, Shiraishi A, Ogawa M, Imozuka K, et al. Detection of MRSA and ESBL-producing bacteria in emergency hospitalized patients [Japanese] [臨牀指針 緊急入院患者における MRSA および ESBL 産生菌の検出状況について]. Jpn J Clin Exp Med. 2012;89(6):813–5.
Kohno J, Kawamura T, Kikuchi A, Akaishi T, Takayama S, Ishii T. A Japanese traditional medicine Hochuekkito promotes negative conversion of vancomycin-resistant Enterococci. Sci Rep. 2021;11(1):11300.
Hanada S, Iwata S, Kishi K, Morozumi M, Chiba N, Wajima T, et al. Host factors and biomarkers associated with poor outcomes in adults with invasive pneumococcal disease. PLoS One. 2016;11(1): e0147877.
Yamagata A, Ito A, Nakanishi Y, Ishida T. Prognostic factors in nursing and healthcare-associated pneumonia. J Infect Chemother. 2020;26(6):563–9.
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985–95.
Hamada Y, Magarifuchi H, Oho M, Kusaba K, Nagasawa Z, Fukuoka M, et al. Clinical features of enterococcal bacteremia due to ampicillin-susceptible and ampicillin-resistant enterococci: an eight-year retrospective comparison study. J Infect Chemother. 2015;21(7):527–30.
Hattori H, Maeda M, Nagatomo Y, Takuma T, Niki Y, Naito Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: a single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–9.
Kainuma A, Momiyama K, Kimura T, Akiyama K, Inoue K, Naito Y, et al. An outbreak of fluoroquinolone-resistant Pseudomonas aeruginosa ST357 harboring the exoU gene. J Infect Chemother. 2018;24(8):615–22.
Yamada K, Imoto W, Yamairi K, Shibata W, Namikawa H, Yoshii N, et al. The intervention by an antimicrobial stewardship team can improve clinical and microbiological outcomes of resistant gram-negative bacteria. J Infect Chemother. 2019;25(12):1001–6.
Hanaoka N, Etoh S, Fujiyoshi N, Hirata T, Omura K. Antimicrobial therapy for multi-drug resistant Pseudomonas aeruginosa (MDRP) infection in patients with severe burns, admitted to Chiba emergency medical center [Japanese] [当医療センターでの重症熱傷患者における多剤耐性緑膿菌感染症に対しての抗菌薬療法]. Jpn J Intensive Care Med. 2013;37(4):311–8.
Nagao M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect. 2013;19(9):852–8.
Ueda S, Okada M, Ito T, Kobayashi K, Takahashi T, Marumo S, et al. Comparative study of the effects of different dosing frequencies of cefmetazole on urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli [Japanese] [基質特異性拡張型 β-ラクタマーゼ産生大腸菌に起因する尿路感染症に対するセフメタゾールの投与回数の違いによる有効性の比較検討]. J Jpn Soc Hosp Pharm. 2022;58(2):167–72.
Mitsuboshi S, Tsuruma N, Watanabe K, Takahashi S, Ito A, Nakashita M, et al. Advanced age is not a risk factor for mortality in patients with Bacteremia caused by extended-spectrum β-lactamase-producing organisms: a multicenter cohort Study. Jpn J Infect Dis. 2020;73(4):288–92.
Tetsuka N, Hirabayashi A, Matsumoto A, Oka K, Hara Y, Morioka H, et al. Molecular epidemiological analysis and risk factors for acquisition of carbapenemase-producing Enterobacter cloacae complex in a Japanese university hospital. Antimicrob Resist Infect Control. 2019;8:126.
Hayakawa K, Nakano R, Hase R, Shimatani M, Kato H, Hasumi J, et al. Comparison between IMP carbapenemase-producing Enterobacteriaceae and non-carbapenemase-producing Enterobacteriaceae: a multicentre prospective study of the clinical and molecular epidemiology of carbapenem-resistant Enterobacteriaceae. J Antimicrob Chemother. 2020;75(3):697–708.
Imai S, Inoue N, Nagai H. Economic and clinical burden from carbapenem-resistant bacterial infections and factors contributing: a retrospective study using electronic medical records in Japan. BMC Infect Dis. 2022;22(1):581.
Furukawa D, Asai S, Nakgohri T. Trend of methicillin - resistant Staphylococcus aureus isolation in gastroenterological surgery] [Japanese] [消化器外科におけるメチシリン耐性黄色ブドウ球菌検出の動向]. J Jpn Soc Surg Infect. 2012;9(2):143–50.
Sano T. MRSA infection of department of respiratory medicine [Japanese] [本学における MRSA 感染症の現状 呼吸器内科における MRSA 感染症の現状]. J Med Soc Toho Univ. 2012;59(6):320–2.
Iwabuchi H, Oguchi K. Actual status of sepsis and countermeasures [Japanese] [4. 敗血症の実態と対策]. J Jpn Soc Dial Ther. 2013;46(2):176–8.
The Japanese Society of Intensive Care Medicine CoSR. 2007 JSICM Sepsis 1st Registry: management of severe sepsis and septic shock in Japan. J Jpn Soc Intensive Care Med. 2013;20(2):329–34.
Nakatsuka Y, Morimoto C, Yasuda I, Tsuji T, Kaji Y, Yasuda T, et al. An analysis of the correlation between guidelines-concordant treatment and the treatment outcome on nursing and healthcare-associated pneumonia patients. Kansenshogaku Zasshi. 2013;87(6):739–45.
Sasaki S, Kuwana Y, Chimori A, Yoshida K. Epidemiological characterization of clinically isolated MRSA in Kinki Central Hospital, mainly for vancomycin MIC [Japanese] [近畿中央病院における MRSA 臨床分離株の疫学調査]. Med J Kinki Central Hosp. 2015;35:25–32.
Kawamura I, Sekiya N, Araoka H, Nei T, Harada S, Kurai H, et al. Surveillance of Methicillin-resistant Staphylococcus aureus in 7 Japanese Hospitals, 2015. Jpn J Infect Prevent Control. 2017;32(3):135–40.
Fukushima M, Maeda A, Soga H, Tomono Y, Machida S, Oi Z, et al. Effects of AST activity on MRSA bacteremia using the automatic simultaneous multi-item genetic testing system (Verigene system) [Japanese] [自動多項目同時遺伝子関連検査システム (Verigeneシステム) を利用した MRSA 菌血症に対する AST 活動の効果]. J Jpn Soc Hosp Pharm. 2022;58(5):545–50.
Mizokami F, Shibasaki M, Yoshizue Y, Noro T, Mizuno T, Furuta K. Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin-resistant Staphylococcus aureus hospital-acquired pneumonia. Clin Interv Aging. 2013;8:1015–21.
Shoji H, Urakawa T, Watanabe K, Hirano T, Katsumi K, Ohashi M, et al. Clinical features, outcomes, and survival factor in patients with vertebral osteomyelitis infected by methicillin-resistant staphylococci. J Orthop Sci. 2016;21(3):282–6.
Isobe M, Uejima E, Seki M, Yamagishi Y, Miyawaki K, Yabuno K, et al. Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother. 2012;18(6):841–7.
Kaku N, Yanagihara K, Morinaga Y, Yamada K, Harada Y, Migiyama Y, et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J Infect Chemother. 2014;20(6):350–5.
Seki M, Takahashi H, Yamamoto N, Hamaguchi S, Ojima M, Hirose T, et al. Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist. 2015;8:113–8.
Shoji H, Maeda M, Shirakura T, Takuma T, Ugajin K, Fukuchi K, et al. More accurate measurement of vancomycin minimum inhibitory concentration indicates poor outcomes in meticillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2015;46(5):532–7.
Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. The economic burden of methicillin-resistant Staphylococcus aureus in community-onset pneumonia inpatients. Am J Infect Control. 2016;44(12):1628–33.
Shime N, Saito N, Bokui M, Sakane N, Kamimura M, Shinohara T, et al. Clinical outcomes after initial treatment of methicillin-resistant Staphylococcus aureus infections. Infect Drug Resist. 2018;11:1073–81.
Uematsu H, Yamashita K, Mizuno S, Kunisawa S, Shibayama K, Imanaka Y. Effect of methicillin-resistant Staphylococcus aureus in Japan. Am J Infect Control. 2018;46(10):1142–7.
Yuasa A, Murata T, Imai K, Yamamoto Y, Fujimoto Y. Treatment procedures and associated medical costs of methicillin-resistant Staphylococcus aureus infection in Japan: a retrospective analysis using a database of Japanese employment-based health insurance. SAGE Open Med. 2019;7:2050312119871181.
Miyazaki T, Yanagihara K, Kakeya H, Izumikawa K, Mukae H, Shindo Y, et al. Daily practice and prognostic factors for pneumonia caused by methicillin-resistant Staphylococcus aureus in Japan: a multicenter prospective observational cohort study. J Infect Chemother. 2020;26(2):242–51.
Umemura Y, Ogura H, Takuma K, Fujishima S, Abe T, Kushimoto S, et al. Current spectrum of causative pathogens in sepsis: a prospective nationwide cohort study in Japan. Int J Infect Dis. 2021;103:343–51.
Tsuzuki S, Yu J, Matsunaga N, Ohmagari N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health. 2021;198:292–6.
Sakamoto Y, Yamauchi Y, Jo T, Michihata N, Hasegawa W, Takeshima H, et al. In-hospital mortality associated with community-acquired pneumonia due to methicillin-resistant Staphylococcus aureus: a matched-pair cohort study. BMC Pulm Med. 2021;21(1):345.
Shimizu M, Mihara T, Ohara J, Inoue K, Kinoshita M, Sawa T. Relationship between mortality and molecular epidemiology of methicillin-resistant Staphylococcus aureus bacteremia. PLoS One. 2022;17(7): e0271115.
Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. Estimating the disease burden of methicillin-resistant Staphylococcus aureus in Japan: retrospective database study of Japanese hospitals. PLoS One. 2017;12(6): e0179767.
Aoyagi T, Kaito C, Sekimizu K, Omae Y, Saito Y, Mao H, et al. Impact of psm-mec in the mobile genetic element on the clinical characteristics and outcome of SCCmec-II methicillin-resistant Staphylococcus aureus bacteraemia in Japan. Clin Microbiol Infect. 2014;20(9):912–9.
Asai N, Sakanashi D, Suematsu H, Kato H, Hagihara M, Nishiyama N, et al. The epidemiology and risk factor of carbapenem-resistant enterobacteriaceae colonization and infections: case control study in a single institute in Japan. J Infect Chemother. 2018;24(7):505–9.
Fukuda H, Sato D, Iwamoto T, Yamada K, Matsushita K. Healthcare resources attributable to methicillin-resistant Staphylococcus aureus orthopedic surgical site infections. Sci Rep. 2020;10(1):17059.
Kashimura N, Kusachi S, Konishi T, Shimizu J, Kusunoki M, Oka M, et al. Impact of surgical site infection after colorectal surgery on hospital stay and medical expenditure in Japan. Surg Today. 2012;42(7):639–45.
Naylor NR, Yamashita K, Iwami M, Kunisawa S, Mizuno S, Castro-Sanchez E, et al. Code-sharing in cost-of-illness calculations: an application to antibiotic-resistant bloodstream infections. Front Public Health. 2020;8: 562427.
Obara H, Saitou J, Fukuda H. Increased burden on medical resources of penicillin-resistant streptococcus pneumoniae infections: estimates using JANIS data. Jpn J Infect Prevent Control. 2015;30(3):165–73.
Saito S, Hayakawa K, Tsuzuki S, Ishikane M, Nagashima M, Mezaki K, et al. A matched case-case-control study of the impact of clinical outcomes and risk factors of patients with IMP-type carbapenemase-producing carbapenem-resistant enterobacteriaceae in Japan. Antimicrob Agents Chemother. 2021;65(3). https://doi.org/10.1128/aac.01483-20.
Uryu K, Nishiura S, Yamamoto T, Umakosi T, Nisida M, Suzuki M, et al. Changes in medical treatment fees for pneumococcal pneumonia in our hospital before and after the introduction of DPC. J Jpn Soc Clin Pathway. 2012;14(2):113–21.
Maeda M, Oto Y, Murayama J, Minemura A, Baba T, Yoshida H, et al. A study of risk factors for antibiotic selective pressure in Carbapenem-treated patients [Japanese] [カルバペネム系薬投与患者における耐性菌選択リスク因子の検討]. J Showa Univ Soc. 2014;74(1):67–72.
Osumi T, Tanaka D, Eguchi T, Imai T, Shimizu H, Sakai M, et al. retrospective study of initiation of empirical therapies out of the antibacterial spectrum for urinary tract infections presumed to be caused by extended spectrum β-lactamases producing bacteria [Japanese] [ESBL 産生菌が原因と推定される尿路感染症にスペクトラムが外れた経験的治療を開始したことが及ぼす影響の後方視的検討]. J Jpn Soc Hosp Pharm. 2021;57(11):1215–20.
Kobayashi H, Moriyama Y, Kurosu H. Report methicillin-resistant Staphylococcus aureus hospital infection surveillance for the fiscal year 2011 [Japanese] [報告 2011年度の Methicillin-resistant Staphylococcus aureus 病院感染症サーベイランス]. Jpn J Infect Prevent Control. 2013;28(3):178–9.
Ogasahara Y, Nagasaki N, Ohno K, Harino T, Yoshida T, Maruko M. Factors causing P. aeruginosa resistance in patients treated with meropenem [Japanese] [Meropenem 投与患者における緑膿菌耐性化因子の検討]. Jpn J Infect Prevent Control. 2012;27(6):419–24.
Kosuge Y, Nagashima G, Enomoto K, Kato A, Noda M, Morishima H, et al. Clinical characteristics of multidrug-resistant bacteria infection with stroke [Japanese] [脳卒中患者における多剤耐性菌感染症の臨床検討]. Neurosurg Emerg. 2013;18(2):173–6.
Fukatsu M, Umemura T, Mizuno T, Ohguchi H, Iwatsu S, Matsumoto S, et al. Positive impacts of antimicrobial stewardship on intial eradication success and drug costs of treatment for clarithromycin-reisistant helicobacter pyloriInfections [Japanese] [薬剤師によるクラリスロマイシン耐性 Helicobacter pylori 感染に対する抗菌薬適正使用支援が初回除菌率と薬剤費に与える影響]. Jpn J Pharm Health Care Sci. 2021;47(11):609–15.
Suzuki T, Tsuchiya M, Niwa T, Watanabe T, Ohta H, Fukako A, et al. Cost effectiveness of controlling healthcare-associated spread of methicillin-resistant Staphylococcus aureus [Japanese] [当院における MRSA 感染制御活動の経済的評価に関する検討]. Jpn J Infect Prevent Control. 2015;30(2):91–6.
Goto Y, Hayashi M, Akasaki J, Ito M, Yamamoto K, Sawa A, et al. Study of risk factors for carriage of vancomycin-resistant enterococcus [Japanese] [バンコマイシン耐性腸球菌の保菌におけるリスク因子の検討]. J Jpn Soc Hospital Pharm. 2014;4:499–502.
Naylor NR, Atun R, Zhu N, Kulasabanathan K, Silva S, Chatterjee A, et al. Estimating the burden of antimicrobial resistance: a systematic literature review. Antimicrob Resist Infect Control. 2018;7:58.
The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2023–2027. 2023. Available from: https://www.mhlw.go.jp/content/10900000/001096228.pdf.
Surveillance of antimicrobial resistance in Europe, 2020 data. 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Surveillance-antimicrobial-resistance-in-Europe-2020.pdf.
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41(1):1–18.
The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016–2020. 2016. Available from: https://www.mhlw.go.jp/content/10900000/0000138942.pdf.
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol. 2005;26(2):166–74.
Thampi N, Showler A, Burry L, Bai AD, Steinberg M, Ricciuto DR, et al. Multicenter study of health care cost of patients admitted to hospital with Staphylococcus aureus bacteremia: impact of length of stay and intensity of care. Am J Infect Control. 2015;43(7):739–44.
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–10.
Poudel AN, Zhu S, Cooper N, Little P, Tarrant C, Hickman M, et al. The economic burden of antibiotic resistance: a systematic review and meta-analysis. PLoS One. 2023;18(5): e0285170.
Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36(1):53–9.
Rodenbeck M, Ayobami O, Eckmanns T, Pletz MW, Bleidorn J, Markwart R. Clinical epidemiology and case fatality due to antimicrobial resistance in Germany: a systematic review and meta-analysis, 1 January 2010 to 31 December 2021. Euro Surveill. 2023. https://doi.org/10.2807/1560-7917.ES.2023.28.20.2200672.
MacKinnon MC, Sargeant JM, Pearl DL, Reid-Smith RJ, Carson CA, Parmley EJ, et al. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):200.
Wozniak TM, Barnsbee L, Lee XJ, Pacella RE. Using the best available data to estimate the cost of antimicrobial resistance: a systematic review. Antimicrob Resist Infect Control. 2019;8:26.
Medical Writing, Editorial, and Other Assistance.
The authors’ heartfelt appreciation goes to Aditya K. Kumar and Anil Dasari from IQVIA India, and Karin Matsumoto and Yawen Dai from IQVIA Solutions Japan for their systematic literature review support; Annoda Kumar and Rosario Vivek from IQVIA India for providing medical writing support; and Todd D. Taylor from IQVIA Solutions Japan for providing editorial assistance; all of which was funded by Pfizer Japan Inc. in accordance with the Good Publication Practice (GPP3) guidelines. (http://www.ismpp.org/gpp3).
Authorship.
All authors met all four requirements for authorship as outlined by the International Committee of Medical Journal Editors (ICMJE). All authors read and approved the final version of the manuscript.
Funding
This work, including the journal’s Rapid Service Fee, was supported by Pfizer Japan Inc.
Author information
Authors and Affiliations
Contributions
Tetsuya Matsumoto, Akira Yuasa, and Naohiro Yonemoto conceptualized and designed the study. Hiroyuki Matsuda and Dilinuer Ainiwaer were responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: Tetsuya Matsumoto has been on the speakers’ bureau for Pfizer Japan Inc., KYORIN Pharmaceutical Co., Ltd., and MSD K.K. Akira Yuasa and Naohiro Yonemoto are full-time employees of Pfizer Japan Inc., and hold stocks and stock options from Pfizer Inc. Hiroyuki Matsuda and Dilinuer Ainiwaer are employees of IQVIA Solutions Japan G.K., which received funding from Pfizer Japan Inc. to undertake the research outlined in this study.
Ethical Approval
Ethics approval was not required for this study. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentation: Akira Yuasa, Tetsuya Matsumoto. Systematic Literature Review on the Clinical and Economic Burdens of Antimicrobial Resistance in the Japanese Population. The 70th East Japan Chapter General Meeting of the Japanese Society of Chemotherapy, October 2023, Tokyo, Japan. Akira Yuasa, Naohiro Yonemoto, Hiroyuki Matsuda, et al. EPH230 Systematic Literature Review on the Clinical and Economic Burdens of Antimicrobial Resistance in the Japanese Population. ISPOR Europe 2023, November 2023, Copenhagen, Denmark.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsumoto, T., Yuasa, A., Matsuda, H. et al. Burden of Antimicrobial Resistance in Japan: A Systematic Literature Review and Meta-Analysis. Infect Dis Ther 13, 1105–1125 (2024). https://doi.org/10.1007/s40121-024-00960-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00960-z