Abstract
W-doped ZnO nanocomposite was easily prepared by sol–gel method. The sample was characterized using a variety of techniques including X-ray diffraction, scanning electron microscopy, transmission electron microscopy, inductively coupled plasma and BET surface area measurement. This reagent can be used as an efficient and heterogeneous catalyst for the preparation of 1, 8-dioxo-octahydroxanthenes from aldehydes in ethanol under mild conditions. The structures of the products were characterized by their physical constants, comparison with authentic samples and IR, 1H NMR and 13C NMR spectroscopy. Easy preparation of the catalyst, mild reaction conditions, easy workup procedure, excellent yields and short reaction times are some advantages of this work. In addition, in this article and for the first time the preparation of 1, 8-dioxo-octahydroxanthenes from the protected derivatives of aldehydes including oximes, semicarbazones and 1,1-diacetates is reported.
Similar content being viewed by others
Background
Xanthenes and their derivatives have received special attention due to their diverse array of biological activities such as anti-inflammatory, antibacterial and antiviral activities [1–3]. Furthermore, these compounds can be used as leuco dyes [4], in laser technology [5] and as pH-sensitive fluorescent materials for the visualization of biomolecular assemblies [6]. Because of their wide range of synthetic, industrial and pharmacological applications, there are several reports in the literature for the synthesis of these types of compounds. 1, 8-Dioxo-octahydroxanthene derivatives are one of the important types of xanthenes that could be easily prepared from the reaction of aromatic aldehydes with dimedone and/or cyclohexadione. Various catalysts were reported for the promotion of this reaction including SbCl3/SiO2 [7], SiO2-R-SO3H [8], MCM-41-SO3H [9], p-dodecylbenzene sulfonic acid [10], triethyl benzyl ammonium chloride [11], diammonium hydrogen phosphate [12] and silica-supported Preyssler nanoparticles [13], silica sulfuric acid [14], Amberlyst-15 [15] and trichloroisocyanuric acid (TCCA) [16] are examples. However, these methods suffer from one or more disadvantages such as: long reaction times, low yields, use of toxic solvents, requirement of the excess amounts of the reagents/catalysts and harsh reaction conditions. Therefore, it is important to find more efficient catalysts and methods for the synthesis of these types of compounds.
Oximes and semicarbazones are used not only for the isolation, purification and characterization, but also for the protection of carbonyl compounds [17, 18]. Since oximes can be prepared from non-carbonyl compounds [19–21], the regeneration of carbonyl compounds from oximes provides an alternative method for the preparation of aldehydes and ketones. In addition, oximes can be used as intermediates for the preparation of nitriles [22–24], nitrones [25], amines [26], amides [27], isoxazoles [28] and chiral α-sulfinyl oximes [29]. Because of their remarkable stability to neutral and basic conditions [30, 31], acylals (1,1-diacetates) have been introduced as the other suitable protection group for aldehydes. In addition, they can be converted into other useful functional groups by reaction with appropriate nucleophiles [32] and used as carbonyl surrogates for asymmetric synthesis [33]. 1,1-Diacetates, on the other hand, are ambient substrates containing two types of reactive carbon centers, the carbon atom of the protected aldehyde function and the carbonyl group in the ester moieties [34]. To the best of our knowledge and in spite of the above-mentioned important applicabilities of oximes, semicarbazones and acylals, there is no report about the preparation of biscoumarins using these types of substrates.
In recent years and because of the unique properties of nanoparticles, synthetic chemists have focused on the synthesis and characterization of these types of catalysts with lower dimensions named as nanocatalysts [35]. Among these types of catalysts, nano metal oxides are widely used in organic reactions, of which zinc oxide as a solid acid catalyst has found considerable applications in different types of important organic transformations [36–44]. To improve the photocatalytic activity of ZnO, many dopant ions (metals and transition metals) were doped into ZnO [45–50]. In 2008, nanosized W-doped TiO2 photocatalysts were synthesized and used for the oxidation degradation of methyl orange by Tian et al. [51]. The results showed that the photocatalytic activity of W-doped TiO2 was much higher than that of the undoped TiO2. They believed that the enhanced photocatalytic efficiency of the W-doped TiO2 can be attributed to the presence of surface acidity. In other words, this catalyst exhibits surface acidity due to the presence of Lewis and Bronsted acidic sites related to W species. In 2013 and on the basis of Ma’s report, Moafi et al. [52] showed that doping of ZnO with 4 % mol of tungsten, in the same manner, can improve the photocatalytic activity of this reagent in the photodegradation of methylene blue.
On the basis of this report, we anticipate that W-doped ZnO nanocomposite can be used as an efficient solid acid catalyst for the speed-up of the reactions which need the use of an acidic catalyst. So, we were interested to investigate the applicability of this reagent in the promotion of the synthesis of 1, 8-dioxo-octahydroxanthenes. Our initial studies clarified that to obtain the best results, the amounts of tungsten should be enhanced to 8 mol%. Therefore, we prepared, identified and applied the % 8 mol W-doped ZnO nano composite in the promotion of the requested reactions.
Methods
General
All chemicals were purchased from Merck, Aldrich and Fluka Chemical Companies and used without further purification. Products were characterized by their physical constants and comparison with authentic samples. The purity determination of the substrates and reaction monitoring were accompanied by TLC using silica gel SIL G/UV 254 plates.
To investigate the morphology of the W-doped sample scanning electron microscopy (SEM) images were obtained on a Philips, XL30. The particles sizes were obtained by transmission electron microscope (TEM) images on a Philips CM10 instrument with an accelerating voltage of 100 kV. Elemental analyses of the samples were carried out by ICP-OES. Measurements were made on an ICP-OES Vista-Pro (Varian) after dissolution of the samples in an HNO3:HF:H2O mixture.
The BET specific surface areas of the synthesized nanocomposite were determined by nitrogen adsorption at liquid nitrogen temperature on a Sibata SA-1100 surface area analyzer. X-ray diffraction measurements were recorded by a Philips PW1840 diffractometer with Cu–Kα radiation, scan rate 0.02 2θ/s and within a range of 2θ of 10°–80° at room temperature.
The IR spectra were recorded on a Perkin Elmer 781 Spectrophotometer. In all the cases, the 1H NMR spectra were recorded with Bruker Avance 300, 400 and 500 MHz instruments. All chemical shifts are quoted in parts per million (ppm) relative to TMS using deuterated solvent. The 13C NMR data were collected on Bruker Avance 100 MHz instrument. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
Catalyst preparation
The W-doped ZnO nanocomposite was prepared by sol–gel method using the precursors of zinc and tungsten [52]. Zinc acetate dihydrate [Zn(Ac)2.2H2O] was used as a zinc oxide source. In a typical procedure, 0.02 mol of zinc acetate dihydrate was dissolved in 50 mL of methanol and heated at 50 °C with stirring for half an hour. Then, certain amounts of sodium tungstate (8 mol% with respect to zinc acetate dihydrate) was dissolved in a mixture of water/methanol [10 mL (2:8)] under vigorous stirring and then the solution was added dropwise into the mixture of zinc acetate dihydrate and methanol, thus making precursor solution A. Afterward, 0.04 mol of sodium hydroxide was dissolved in 50 mL of methanol and heated at 50 °C with stirring for 1 h, making precursor solution B. To make ZnO nano-sol, the solution of sodium hydroxide (solution B) was added dropwise into the solution A under constant stirring for half an hour and then the mixture was heated at 50 °C for a further half an hour. Subsequently, a homogenous sol was obtained. The obtained solution was precipitated after continuous stirring for 2 h and cooling to room temperature. After 24 h, the colloidal solution was washed several times with methanol. Finally, the obtained precipitate was dried at 80 °C and then calcinated at 300 °C for 3 h. Similarly, 2, 4 and 6.0 mol% W-doped ZnO and undoped ZnO samples were also prepared by repeating the above procedure. The experimental results showed that the W-ZnO with 8 mol% W has the highest catalytic activity.
General procedure for the synthesis of 1, 8-dioxo-octahydroxanthenes
A mixture of dimedone and/or cyclohexadione (2 mmol), aldehyde and/or protected aldehyde (1 mmol) and W-ZnO (5 mg) in ethanol (3 mL) was stirred at room temperature and/or refluxing conditions for the appropriate time. After completion of the reaction [monitored by TLC: EtOAc: n-hexane (2:8)], hot ethanol (2 mL) was added and the mixture was filtered to separate the catalyst. Then the crude product was recrystallized from EtOH:H2O (95:5) to afford the pure product.
Results and discussion
Catalyst characterization
Powder X-ray diffraction
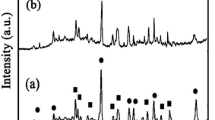
Figure 1 shows the XRD patterns of ZnO and W-doped ZnO nanocomposite. The W-doped ZnO nanocomposite showed a hexagonal wurtzite crystal structure and high crystallinity of ZnO. The peaks at 31.7º, 34.5º, 36.7º, 47.7º, 56.5º, 62.9º and 67.9º 2θ are associated with the (100), (002), (101), (102), (110), (103) and (112) planes of the ZnO hexagonal wurtzite structure. The diffraction peaks of the W-ZnO are broad, indicating a small crystal size of this sample. The XRD pattern of the W-ZnO catalyst shows that there is no major change in the hexagonal crystal structure of ZnO after the tungsten-doping process. However, it can be indicated that W+6 ions are uniformly dispersed on ZnO nanoparticles in the form of highly dispersed WO3 clusters. Meanwhile, the ionic radius of the dopant ion is the most important factor, which strongly influences the ability of the dopant to enter into the ZnO crystal lattice. If the ionic radius of the doping metal ions matches those of the lattice metal ion in oxides, the doping metal ion will substitute itself for the lattice in the doping reactive process (substitutional mode). While if the ionic radius of the dopant is much bigger or smaller than that of Zn2+, the dopant substituting for ZnO crystal lattice ions results in crystal lattice distortion. The ionic radius of W6+ (60 pm) is smaller or closer than that of Zn2+ ion (74 pm). Therefore, it would be possible that some W6+ ions replace the lattice Zn2+ ions and thus occupy the lattice Zn2+ positions [52].
There were no detectable peaks relating to the existence of a separate dopant metal phase in any corresponding pattern. This could be attributed to the fact that the dopant metals/metal oxides were too low in concentration and/or amorphous structure to be seen as a separate phase. The real W content in W-ZnO sample was measured by ICP-OES (Table 1). The weight ratio of W/Zn in the W-ZnO nanocomposite was 0.228 %.
Surface area and pore distribution measurements
The surface area of the catalyst is the most important factor influencing catalytic activity. The surface area of W-ZnO nanocomposite was determined using the nitrogen gas adsorption method. The BET surface area of the prepared W-ZnO yielded a relatively high surface area (93.70 m2/g).
SEM analysis
The surface morphology and dispersion of the sample were determined by scanning electron microscopy (SEM). Figure 2 shows SEM micrographs of 8 mol% W-ZnO. The image reveals that the particles in this sample have a relatively sphere-like morphology and the nanoparticles were composed of agglomerates of fine W-doped ZnO nanoparticles with particle size <100 nm. The small and uniform size of the prepared reagent can affect its catalytic performance.
TEM analysis
Figure 3 depicts transmission electron micrograph of 8 mol% W-ZnO. The TEM image of W-ZnO shows that the sample consists of fine particles with diameters <20 nm in size. On the other hand, the size of the W-ZnO nanoparticles was observed as aggregation of fine nanoparticles with average particle size of 10–15 nm (Table 1).
Catalytic activity
In recent years a considerable amount of our research program has focused on the development of new methods and use of new reagents for the synthesis of xanthene derivatives [53–58]. Herein and in continuation of these studies, we wish to report the preparation of % 8 mol W-doped ZnO nanocomposite and its applicability as a new and efficient catalyst in the promotion of the synthesis of 1, 8-dioxo-octahydroxanthenes under mild conditions. After preparation and identification of W-doped ZnO nanocomposite (as reported in the experimental section), its application in the promotion of the synthesis of 1, 8-dioxo-octahydroxanthene is studied. To optimize the reaction conditions, the reaction of 4-chlorobenzaldehyde (1 mmol) with dimedone (2 mmol) was studied in different solvents and under solvent-free conditions at different temperatures (Table 2). Also, the effect of the catalyst load on the model reaction was studied. The results are shown in Fig. 4.
On the basis of the obtained results, it is concluded that the best results can be obtained under the conditions shown in Scheme 1.
To assess the efficiency of W-ZnO in the preparation of 1, 8-dioxo-octahydroxanthenes, various aromatic aldehydes were reacted with 1, 3-cyclohexanedione and/or dimedone under optimal reaction conditions (Table 3). These results show that the requested reactions efficiently occurred with excellent yields in very short times (Table 3). It seems that the electronic nature of the functional group on the ring of the aldehyde exerted a slight influence on the reaction time.
After the above-mentioned studies, we were interested in investigating the applicability of the same method to the preparation of 1, 8-dioxo-octahydroxanthenes from the protected aldehydes (e.g., oximes, semicarbazones and 1,1-diacetates). Our investigations clarified that using this method the best results can be obtained when the reaction was conducted in refluxing ethanol (Scheme 2).
After optimization of the reaction conditions, different types of protected derivatives of aldehydes were efficiently converted to the requested 1, 8-dioxo-octahydroxanthenes under the selected conditions (Table 4). All reactions were performed under completely heterogeneous conditions during relatively short times with good to high yields.
A plausible mechanism for the synthesis of 1, 8-dioxo-octahydroxanthenes catalyzed by W-ZnO is shown in Scheme 3.
To illustrate the efficiency of the present method, Table 5 compares some of our results obtained from the synthesis of xanthene derivatives with the same results reported by the other research groups. These results indicated that in most cases, the reactions were performed under heated conditions using larger amounts of other catalysts.
It is important to note that the reaction in the presence of Fe3O4 or ZnO-NPs needs larger amounts of catalysts at high temperatures (Table 5, entries 8–10), while in the present method the reaction is carried out under very mild conditions using few amounts of W-doped ZnO nanocomposite. In addition, to compare the applicability and efficiency of W-ZnO with other catalysts, we have tabulated the TOF (turnover frequency) of these catalysts in this reaction. Clearly, W-ZnO is superior in terms of TOF to the compared catalysts.
According to literature [44], it can be concluded that the catalytic efficiency of W-ZnO can be attributed to the presence of surface acidity. W/ZnO catalysts exhibit surface acidity due to the presence of Lewis and Bronsted acidic sites related to W6+ species. Thus, the acidic surface of W-ZnO has a higher affinity for reactive species.
Conclusion
In summary, we have introduced W-doped ZnO nanocomposite as a highly efficient nanocatalyst for the promotion of the synthesis of 1, 8-dioxo-octahydroxanthenes under mild and completely heterogeneous reaction conditions. This method has several advantages such as ease of preparation and handling of the catalyst, easy workup and procedure, high reaction rates and excellent yields. Also, and for the first time, different types of protected aldehydes were successfully employed in these types of reactions and the corresponding products were obtained in high to excellent yields.
References
Chatterjee, S., Iqbal, M., Kauer, J.C., Mallamo, J.P., Senadhi, S., Mallya, S., Bozyczko-Coyne, D., Siman, R.: Xanthene derived potent nonpeptidic inhibitors of recombinant human calpain. Bioorg. Med. Chem. Lett. 6, 1619–1622 (1996)
Vieira, E., Huwyler, J., Jolidon, S., Knofach, F., Mutel, V., Wichmann, J.: 9H-xanthene-9-carboxylic acid [1, 2, 4]oxadiazol-3-yl- and (2H-tetrazol-5-yl)-amides as potent, orally available mGlu1 receptor enhancers. Bioorg. Med. Chem. Lett. 15, 4628–4631 (2005)
Hafez, H.N., Hegab, M.I., Ahmed-Farag, I.S., El-Gazzar, A.B.A.: Facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1, 3, 4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 18, 4538–4543 (2008)
Kitahara, Y., Tanaka, K.: Synthesis, crystal structure and properties of thiaheterohelicenes containing phenolic hydroxy functions. Chem. Commun. 9, 932–933 (2002)
Ahmad, M., King, T.A., Ko, D.K., Cha, B.H., Lee, J.: Performance and photostability of xanthene and pyrromethene laser dyes in sol–gel phases. J. Phys. D Appl. Phys. 35, 1473–1476 (2002)
Knight, C.G., Stephens, T.: Xanthene-dye-labelledphosphatidylethanolamines as probes of interfacial pH. J. Biochem. 258, 683–689 (1989)
Zhang, Z.H., Liu, Y.H.: Antimony trichloride/SiO2 promoted synthesis of 9-aryl-3,4,5,6,7,9-hexahydroxanthene-1,8-diones. Catal. Commun. 9, 1715–1719 (2008)
Mahdavinia, G.H., Bigdeli, M.A., Hayeniaz, Y.S.: Covalently anchored sulfonic acid on silica gel (SiO2–R–SO3H) as an efficient and reusable heterogeneous catalyst for the one-pot synthesis of 1,8-dioxo-octahydroxanthenes under solvent-free conditions. Chinese Chem. Lett. 20, 539–541 (2009)
Rostamizadeh, S., Amani, A.M., Mahdavinia, G.H., Amiri, G., Sepehrian, H.: Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media. Ultrason. Sonochem. 17, 306–309 (2010)
Jin, T.S., Zhang, J.S., Xiao, J.C., Wang, A.Q., Li, T.S.: Clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenzenesulfonic acid in aqueous media. Synlett 5, 866–870 (2004)
Wang, X.S., Shi, D.Q., Li, Y.L., Chen, H., Wei, X.Y., Zong, Z.M.: A clean synthesis of 1-dioxo-hexahydro- xanthene derivatives in aqueous media catalyzed by TEBA. Synth. Commun. 35, 97–104 (2005)
Darvish, F., Balalaei, S., Chadegani, F., Salehi, P.: Diammonium hydrogen phosphate as a neutral and efficient catalyst for synthesis of 1, 8-dioxo-actahydroxanthene derivatives in aqueous media. Synth. Commun. 37, 1059–1067 (2007)
Javid, A., Heravi, M.M., Bamoharram, F.F.: One-pot synthesis of 1,8-dioxo-octahydro xanthenes utilizing silica-supported preyssler nano particles as novel and efficient reusable heterogeneous acidic catalyst. E J. Chem. 8, 910–916 (2011)
Mozhdeh, S., Peiman, M., Ayoob, B.: Solvent-free synthesis of aryl-14H-dibenzo[a, j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst. Dyes Pig. 76, 836–839 (2008)
Das, B., Thirupathi, P., Mahender, I., Saidi Reddy, V., Koteswara Rao, Y.: Iodine catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo[a, j]xanthenes. J. Mol. Catal. A Chem. 255, 74–77 (2006)
Bigdeli, M.A., Nemati, F., Mahdavinia, G.H., Doostmohammadi, H.: A series of 1,8-dioxo-octahydro-xanthenes are prepared using trichloroisocyanuric acid. Chinese Chem. Lett. 20, 1275–1278 (2009)
Greene, T.W., Wuts, P.G.M.: Protective groups in organic synthesis, 3rd edn. Wiley, New York (1991)
Curini, M., Rostai, O., Pisani, E.: Solvent-free synthesis of aryl-14H-dibenzo[a,j]xanthenes and 1,8-dioxo-octahydro-xanthenes using silica sulfuric acid as catalyst. Synlett 333–336 (1996)
Kabalka, G.W., Pace, R.D., Wadgaonkar, P.P.: One-pot synthesis of aryl 14H-dibenzo[a, j]xanthene leuco-dye derivatives. Synth. Commun. 20, 2453–2485 (1990)
Fujisawa, T., Kurita, Y., Sato, T.: A convenient synthesis of ketoximes from grignard reagents and nitro compounds activated by N,N-dimethyl chloromethyleniminium chloride. Chem. Lett. 10, 1537–1540 (1983)
Barton, D.H.R., Beaton, J.M., Geller, L.E., Pechet, M.M.: Contribution x from the research institute for medicine and chemistry, Cambridge, mass. A new photochemical reaction. J. Am. Chem. Soc. 83, 4076–4083 (1961)
Lee, K., Han, S.B., Yoo, E.M., Chung, S.R., Oh, H., Hong, S.: Efficient transformation of aldoximes to nitriles using 2-chloro-1-methylpyridinium iodide under mild conditions. Synth. Commun. 34, 1775–1782 (2004)
Sarvari, M.S.: ZnO/CH3COCl: a new and highly efficient catalyst for dehydration of aldoximes into nitriles under solvent-free condition. Synthesis 34, 787–790 (2004)
Movassagh, B., Fazeli, A.: Direct synthesis of aromatic nitriles from aldehydes using hydroxyl amine and oxalyl chloride. Synth. Commun. 37, 623–628 (2007)
Schoenewalat, E.F., Kinnel, R.B., Davis, P.: Improved synthesis of anti-benzaldoxime. Con comitant cleavage and formation of nitriles. J. Org. Chem. 33, 4270–4272 (1968)
Abiraj, K. Channe, G.D.: Zinc/ammonium formate: a new facile system for the rapid and selective reduction of oximes to amines. J. Chem. Res. (S) 332–234 (2003)
Ghiaci, M., Hassan, I.G.: A facile Beckmann rearrangement of oximes with AlCl3 in the solid state. Synth. Commun. 28, 2275–2280 (1998)
Peter, W., Joan, M.F., Laura, S.: Microwave promoted oxazole synthesis: cyclocondensation cascade of oximes and acyl chlorides. Tetrahedron Lett. 46, 5463–5466 (2005)
Hajipour, A.R., Mahboubghah, N.J.: 1-Benzyl-4-aza-1-azoniabicyclo[2.2.2]octane periodate: a mild and efficient oxidant for the cleavage of oxime double bonds under anhydrous conditions. J. Chem. Res. (S) 122–123 (1998)
Greene, T.W., Wuts, P.G.M.: Protective groups in organic synthesis, 3rd edn. Wiley, Hoboken (1999)
Gregory, M.J.: Evidence for a cyclic AA11 mechanism in the hydrolysis of benzylidene diacetates. J. Chem. Soc. B 1201–1207 (1970)
Heerden, F.R., Huyser, J.J., Williams, D.B.G., Holzapfer, C.W.: Palladium-catalysed substitution reactions of geminal allylic diacetates. Tetrahedron Lett. 39, 5281–5284 (1998)
Trost, B.M., Lee, C.: Gem-diacetates as carbonyl surrogates for asymmetric synthesis. Total syntheses of sphingofungins E and F. J. Am. Chem. Soc. 123, 12191–12201 (2001)
Sandberg, M., Sydnes, L.K.: The chemistry of acylals Part II Formation of nitriles by treatment of acylals with trimethyl silyl azide in the presence of a Lewis acid. Tetrahedron Lett. 39, 6361–6364 (1998)
Shirini, F., Abedini, M.: Application of nanocatalysts in multi-component reactions. J. Nanosci. Nanotechnol. 13, 4838–4860 (2013)
Safaei-Ghomi, J., Ghasemzadeh, M.A.: Zinc oxide nanoparticles: a highly efficient and readily recyclable catalyst for the synthesis of xanthenes. Chinese Chem. Lett. 23, 1225–1229 (2012)
Maghsoodlou, M.T., Habibi-Khorassani, S.M., Shahkarami, Z., Maleki, N., Rostamizadeh, M.: An efficient synthesis of 2,2′-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) and 1,8-dioxo-octahydroxanthenes using ZnO and ZnO-acetyl chloride. Chinese Chem. Lett. 21, 686–689 (2010)
Tamaddon, F., Sabeti, M.R., Jafari, A.A., Tirgir, F., Keshavarz, E.: ZnO and ZnO-nanoparticles: efficient and reusable heterogeneous catalysts for one-pot synthesis of N-acylsulfonamides and sulfonate esters. J. Mol. Catal. A Chem. 351, 41–45 (2011)
Paul, S., Bhattacharyya, P., Das, A.R.: One-pot synthesis of dihydropyrano[2,3-c]chromenes via a three component coupling of aromatic aldehydes, malononitrile and 3-hydroxycoumarin catalyzed by nano-structured ZnO in water: a green protocol. Tetrahedron Lett. 52, 4636–4641 (2011)
Ghosh, P.P., Das, A.R.: Nano crystalline ZnO: a competent and reusable catalyst for one-pot synthesis of novel benzylaminocoumarin derivatives in aqueous media. Tetrahedron Lett. 53, 3140–3143 (2012)
Bhattacharyya, P., Pradhan, K., Paul, S., Das, A.R.: Nano crystalline ZnO catalyzed one-pot multicomponent reaction for an easy access of fully decorated 4H-pyran scaffolds and its rearrangement to 2-pyridone nucleus in aqueous media. Tetrahedron Lett. 53, 4687–4691 (2012)
Kassaee, M.Z., Movahedi, F., Masrouri, H.: ZnO nanoparticles as an efficient catalyst for the one-pot synthesis of α-amino phosphonates. Synlett 8, 1326–1330 (2009)
Dharma Rao, G.B., Kaushik, M.P., Halve, A.K.: An efficient synthesis of naphtha[1,2-e]oxazinone and 14-substituted-14H-dibenzo[a, j]xanthene derivatives promoted by zinc oxide. Tetrahedron Lett. 53, 2741–2744 (2012)
Kassaee, M.Z., Masrouri, H., Movahedi, F.: ZnO-nanoparticle-promoted synthesis of polyhydroquinoline derivatives via multicomponent Hantzsch reaction. Monatsh. Chem. 141, 317–322 (2010)
Sun, J.H., Dong, S.Y., Feng, J.L., Yin, X.J., Zhao, X.C.: Enhanced sunlight photocatalytic performance of Sn-doped ZnO for methylene blue degradation. J. Mol. Catal. A Chem. 335, 145–150 (2011)
Zheng, Y.Z., Tao, X., Hou, Q., Wang, D., Zhou, W.L., Hui, X., Qian, H., Chen, J.F.: Novel ZnO-based film with double light-scattering layers as photoelectrodes for enhanced efficiency in dye-sensitized solar cells. Chem. Mater. 22, 928–934 (2010)
Patil, A.B., Patil, K.R., Pardeshi, S.K.: Ecofriendly synthesis and solar photocatalytic activity of S-doped ZnO. J. Hazard. Mater. 183, 315–328 (2010)
Wu, C., Shen, L., Yu, H., Huang, Q., Zhang, Y.C.: Synthesis of Sn-doped ZnO nanorods and their photo catalytic properties. Mater. Res. Bull. 46, 1107–1112 (2011)
Karunakaran, C., Gomathisankar, P., Manikandan, G.: Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 123, 585–594 (2010)
Barick, K.C., Singh, S., Aslamb, M., Bahadur, D.: Porosity and photocatalytic studies of transition metal doped ZnO nanoclusters. Micro. Meso. Mater. 134, 195–202 (2010)
Tian, H., Ma, J., Li, K., Li, J.: Photocatalytic degradation of methyl orange with W-doped TiO2 synthesized by a hydrothermal method. Mater. Chem. Phys. 112, 47–51 (2008)
Fallah Moafi, H., Zanjanchi, M.A., Fallah Shojaie, A.: Tungsten-doped ZnO nanocomposite: synthesis, characterization, and highly active photocatalyst toward dye photodegradation. Mater. Chem. Phys. 139, 856–864 (2013)
Shirini, F., Khaligh, N.G.: Succinimide-N-sulfonic acid: an efficient catalyst for the synthesis of xanthenes derivatives under solvent-free conditions. Dyes Pig. 95, 789–794 (2012)
Shirini, F., Abedini, M., Pourhasan, R.: N-sulfonic acid poly(4-vinylpyridinium) chloride: a novel polymeric and reusable catalyst for the preparation of xanthenes derivative. Dyes Pig. 99, 250–255 (2013)
Shirini, F., Akbari-Dadamahaleh, S., Mohammad-Khah, A.: Rice-husk-supported FeCl3 nano-particles: introduction of a mild, efficient and reusable catalyst for some of the multi-component reactions. C. R. Chimie. 16, 945–955 (2013)
Shirini, F., Imanzadeh, G.H., Abedini, M., Akbari Dokhte-Ghaziani, M., Ghods Ghasemabadi, P., Safarpoor Langroodi, M.: Introduction of two efficient catalysts for the synthesis of 1,8-dioxo-octahydroxanthene derivatives in the absence of solvent. Iran J. Catal. 2, 115–119 (2012)
Shirini, F., Yahyazadeh, A., Mohammadi, K.: One-pot synthesis of various xanthene derivatives using ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable catalyst under solvent-free conditions. Chinese Chem. Lett. 25, 341–347 (2014)
Shirini, F. Abedini, M. Akbari-Dadamahaleh, S. Rahmaninia, A.: Iranian chemist’s efforts to provide various effective methods for the synthesis of xanthenes. J. Iran Chem. Soc. 2013. doi:10.1007/s13738-013-0353-y
Niknam, K., Damya, M.: 1-Butyl-3-methylimidazolium hydrogen sulfate [bmim]HSO4: an efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydro xanthenes. J. Chinese Chem. Soc. 56, 659–665 (2009)
Ilangovan, A., Muralidharan, S., Sakthivel, P., Malayappasamy, S., Karuppusamy, S., Kaushik, M.P.: Simple and cost effective acid catalysts for efficient synthesis of 9-aryl-1,8-dioxo-octahydro xanthene. Tetrahedron Lett. 54, 491–494 (2013)
John, A., Yadav, P.J.P., Palaniappan, S.: Clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by polyaniline-p-toluenesulfonate salt in aqueous media. J. Mol. Catal. A Chem. 248, 121–125 (2006)
Shaterian, H.R., Hosseinian, A., Ghashang, M.: Ferric hydrogen sulfate as an efficient heterogeneous catalyst for environmentally friendly greener synthesis of 1,8-dioxo-octahydroxanthenes. Turk. J. Chem. 33, 233–240 (2009)
Zhou, Z., Deng, X.: [Et3NH][HSO4] catalyzed efficient and green synthesis of 1,8-dioxo-octahydroxanthenes. J. Mol. Catal. A Chem. 367, 99–102 (2013)
Zare, A., Moosavi-Zare, A.R., Merajoddin, M., Zolfigol, M.A., Hekmat-Zadeh, T., Hasaninejad, A., Khazaei, A., Mokhlesi, M., Khakyzadeh, V., Derakhshan-Panah, F.H., Beyzavi, M., Rostami, E., Arghoon, A., Roohandeh, R.: Ionic liquid triethylamine-bonded sulfonic acid [Et3N-SO3H]Cl as a novel, highly efficient and homogeneous catalyst for the synthesis of β-acetamido ketones, 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a, j]xanthenes. J. Mol. Liq. 167, 69–77 (2012)
Zare, A., Mokhlesi, M., Hasaninejad, A.R., Hekmat-Zadeh, T.: Solvent-free synthesis of 1,8-dioxo-octahydroxanthenes and 14-aryl-14H-dibenzo[a, j]xanthenes using saccharin sulfonic acid as an efficient and green catalyst. E J. Chem. 9, 1854–1863 (2012)
Ghasemzadeh, M.A., Safaei-Ghomi, J., Zahedi, S.: Fe3O4 nano-particles: a highly efficient and easily reusable catalyst for the one-pot synthesis of xanthene derivatives under solvent-free conditions. J. Serb. Chem. Soc. 78, 769–779 (2013)
Acknowledgments
The authors are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Arbosara, F.S., Shirini, F., Abedini, M. et al. Introduction of a new high yielding method for the synthesis of 1, 8-dioxo-octahydroxanthenes using W-doped ZnO nanocomposite. J Nanostruct Chem 5, 55–63 (2015). https://doi.org/10.1007/s40097-014-0134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0134-x