Abstract
L10-FePt nanoparticles with different Ag percentages were prepared. The presence of 6% Ag in co-sputtering process with deposition rate of 1 \( {\dot{A}}/\text{s} \) at 500 °C led to the formation of FePt nanoparticles with 5–7 nm size in L10-fct phase. Moreover, Ag atoms laying in boundaries of these nanoparticles in these circumstances caused c axis to orient and (001) peak intensity in XRD spectrum to increase. This is the result of the indirect exchange interactions of Fe magnetic atoms through Ag atoms in these boundaries. Therefore, where L10-FePt grains have the most boundaries with each other, the order index would be maximum.
Similar content being viewed by others
Introduction
The (001) and (110) peaks are appeared in FePt samples with the formation of L10-fct compound ordering. The intensity of these peaks is lower than that of (111) peak [1]. However, the fcc structure factors for these peaks are zero. When the c axis of L10-fct nanocrystals align vertical to the surface of FePt layer, the (001) peak intensity increases with respect to other peak’s intensity [2].
The presence of Ag in FePt granular layer decreases the L10-fct phase transition temperature [3, 4]. As a result, it provides the possibility of nanoparticles formation with controlled size [5]. Moreover, it causes nanocrystal c axes of L10-fct structure to be paralleled and (001) peak intensity to increase [6, 7]. At high temperature, in the first place, Ag atoms separate from prior deposited Ag sublayer and enter to FePt crystal lattice, but gradually after their increasing they leave the lattice to form their own distinct crystal lattice, and at least part of them will form FePt–Ag nanocomposite [7].
The intense uniaxial magnetic anisotropy in L10-fct phase of FePt nanoparticles is due to indirect exchange interaction [8, 9] between Fe magnetic atoms through Pt nonmagnetic atoms in c crystal direction [10].
Appropriate selecting of Ag or Cu percentage can lead to L10-FePt nanoparticles formation and orientation with suitable size [11,12,13,14]. In present work, the variation in (001) to (111) peak intensity ratio as an indicator of orientation amount was measured with respect to different Ag percentages. It has been shown that this alteration could be the result of indirect exchange interaction between L10 phase FePt nanoparticles through Ag atoms. The synthesis method of nanocomposites was co-sputtering at 500 °C.
Experimental details
FePt granular nanolayers with the thickness of 10 nm have been deposited on Si substrate using rf and dc co-sputtering method. The temperature of substrates has been increased up to 500 °C during deposition process. The base pressure of chamber at the beginning of the process was \( 10^{ - 5} \) Torr which was \( 10^{ - 2} \) Torr for Ar. The applied rf power was 74 w. dc voltage of 242–244 V which was used for Ag deposition. Ag percentages of six prepared samples which were named as samples 1–6 were 0%, 3%, 4%, 6%, 11%, and 20%, respectively.
X-ray diffraction (XRD) studies were performed by a STOE STADI MP model using the wave length of 1.54 Å. The magnetic properties of the samples were investigated using a vibrating sample magnetometer, (VSM), 7400 Lake Shore model. Conventional FE-SEM studies were carried out using a FE-SEM model MIRA3 Tescan working at 15.0 kV.
Results
Figure 1 shows the EDS analysis of sample 2 with 3% Ag. According to these results, the composition of the samples is Fe40Pt60 as nanocomposite with different percentages of Ag.
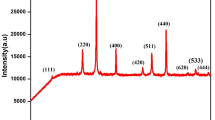
The XRD patterns of samples with different Ag percentages are shown in Fig. 2. The XRD patterns of the samples 1 and 6 are shown in Fig. 2a and b in which Ag concentrations are 0% and 20%, respectively. The first effect of the Ag presence is the appearance of the (001) peak in the XRD pattern of FePt nanoparticles which specifically belongs to the L10-fct structure. FePt sample peaks are wide, and those are narrow for Ag (Fig. 2b). It shows that 20% of Ag causes larger Ag grains to form separately from smaller FePt grains. The second effect of the Ag presence is that for FePt–Ag nanoparticles with L10-FePt structure, (001) peak has shifted positively for 1° and it appears at 2θ = 25° (Fig. 2c–f). This is due to the indirect exchange interaction in these nanoparticles. The parallel research has been conducted on this effect, and the results will be published in other manuscript. The third effect of the presence of Ag would be that c crystal directions of the FePt nanoparticles are aligned (Fig. 2d). The proportion of (001) to (111) peak intensity [I(001)/I(111)] in XRD pattern of samples is an indicator for such alignment. The change of this indicator is shown in Fig. 2b–f, and its variation in terms of Ag percentages is shown in Fig. 3. According to this figure, in co-sputtering deposition on Si substrate in 500 °C, the 6% of the Ag presence leads to the maximum crystal alignment of L10-FePt nanoparticles.
FE-SEM analyses of samples 2, 4, 6 are shown in Fig. 4 where the figure for Ag layer has been illustrated for comparison. According to 4a, in sample 6 with 20% of Ag, two grain categories with different sizes have formed. The first category is Ag grains with average size of 40 nm, and the second one is FePt grains with size of 10–20 nm. With this amount of Ag, during sputtering deposition on hot Si substrate, Ag forms separate crystal lattice (Figs. 4a and 2b). On the other hand, there is not any separate Ag nanoparticles shown in Fig. 4b and c; however, narrow Ag peaks in XRD patterns are the evidence for the existence of such Ag in samples 2–5 (Fig. 2f–c). It is obvious that a thin layer of Ag has been formed under the FePt nanoparticles. In sample 4 (Figs. 2d and 4b), the 5–7 nm FePt nanoparticles have been deposited on the thin layer of Ag. The lower percentage of Ag (3%) in sample 2 causes defects which are shown in Fig. 4c. For comparison, FE-SEM image of Ag nanoparticles in monolayer of Ag is shown in Fig. 4d. This sample has been sputtered in room temperature.
The VSM analyses of sample 4, which is 5–7 nm nanoparticles with 6% Ag, are shown in Fig. 5. These analyses were carried in easy axis (E.A) and hard axis (H.A) directions. The difference in the hysteresis loops of the sample such as different coercivity and remanent magnetization confirms that it has been oriented in the presence of Ag.
Discussion and conclusion
The crystal structure of Ag sublayer is formed faster than that of FePt, and it causes Ag grains to grow faster. The Ag narrow peaks presence in Fig. 2c–f is the reason for simultaneous thin Ag layer formation underneath FePt nanoparticles. In the presence of 6% of Ag during co-sputtering at 500 °C, the uniform layer of FePt nanoparticles with 5–7 nm in size has been deposited on thin layer of Ag (Fig. 4b). The presence of 3% of Ag has led to formation of granular and ununiform sublayer, so some cracks were appeared on FePt surface (Fig. 4c). Moreover, due to the presence of 20% of Ag, in these conditions, the size of Ag and FePt grains would be 40 nm and 10–20 nm, respectively (Fig. 4a).
The following discussion confirms that the Fe–Ag–Fe indirect exchange interaction through Ag, between Fe atoms in the boundaries of the neighbor nanoparticles, causes crystal c axis of these nanoparticles to align in the same direction and the intensity of (001) peak to increase (Fig. 2d).
The maximum amount of I(001)/I(111) index according to the XRD results, which is shown in Fig. 3, was obtained for 6% of Ag. As shown in Fig. 4b, the more tiny FePt grain boundaries, the more Ag atoms laying possibility and indirect exchange interaction would be. On the other hand, with 20% of Ag, large grains of Ag are formed and regions between FePt nanoparticles boundaries become empty of Ag atoms. In consequence, as it is shown in Fig. 3, the easy axis order index decreases. Sample 2 with 3% Ag also has less Ag atoms in FePt nanoparticles grain boundaries and I(001)/I(111) index decreases. Therefore, Fe and Pt with 6% Ag co-sputtering at 500 °C with thickness of 10 nm and deposition rate of 1 \( {\dot{A}}/\text{s} \) would be the optimum conditions for FePt nanoparticles synthesis.
References
Wang, H., Shang, P., Zhang, J., Guo, M.W., Mu, Y., Li, Q., Wang, H.: One-step synthesis of high-coercivity L10-FePtAg nanoparticles: effects of Ag on the morphology and chemical ordering of FePt nanoparticles. Chem. Mater. 25, 2450–2454 (2013)
Li, Y.B., Lou, Y.F., Zhang, L.R., Ma, B., Bai, J.M., Wei, F.L.: Effect of magnetic field annealing on microstructure and magnetic properties of FePt films. J. Magn. Magn. Mater. 322, 3789–3791 (2010)
Iskandar, F., Lee, H.M., Toda, T., Iwaki, T., Okuyama, K.: Fabrication of L10 FePtAg nanoparticles and a study of the effect of Ag during the annealing process. J Magn Magn Mater 305, 514–519 (2006)
Xu, X.H., Wu, H.S., Wang, F., Li, X.L.: Structure and magnetic properties of FePt and FePt/Ag thin films deposited by magnetron sputtering. Thin solid Film 472, 222–226 (2005)
Ikeda, R., Kagami, M., Kato, T., Iwata, S., Tsunashima, S.: Perpendicular anisotropy and microstructure of MBE-grown FePt–Ag and FePt–MgO granular films. J. Magn. Soc. Jpn 33, 493–497 (2009)
Wang, H., Zhang, J., Mo, Q., Yang, F.J., Wang, H.B., Miao, X.S.: Microstructure and magnetic properties of (0 0 1)-oriented L10 FePt films: role of Ag underlayer and Fe/Pt ratio. Mater. Res. Bull. 47, 2974–2976 (2012)
Roghani, R., Sebt, S.A., Khajehnezhad, A.: High magnetic coercivity of FePt–Ag/MgO granular nanolayers. Phys. C (Amsterdam, Neth.) 549, 15–17 (2018)
Stiles, M.D.: Interlayer exchange coupling. J. Magn. Magn. Mater. 200, 322–337 (1999)
Crisan, A.D., Vasiliu, F., Mercioniu, I., Crisan, O.: Role of Ag addition in L 10 ordering of FePt-based nanocomposite magnets. Philos. Mag. 94, 174–189 (2014)
Kundu, A., Zhang, S.: Temperature dependence of RKKY interaction. J. Magn. Magn. Mater. 393, 331–333 (2015)
Granz, S.D., Barmak, K., Kryder, M.H.: Granular L10 FePt–B and FePt–B–Ag (001) thin films for heat assisted magnetic recording. J. Appl. Phys. 111, 07b709 (2012)
Dong, K., Cheng, X., Cheng, W., Chen, S., Yang, X.: Fabrication and magnetic properties of Ag/FePt thin films. Mater. Manuf. Process. 27, 1160–1163 (2012)
Scheibel, F., Hearing, F., Ziemann, P., Wiedwald, U.: Role of developing L10 chemical order on the (0 0 1)-texture formation of (Fe1-xCux) Pt films grown on amorphous substrates. J. Phys. D Appl. Phys. 48, 085001 (2015)
Kaushik, N., Sharma, P., Tanaka, S., Makino, A., Esashi, M.: Preferentially oriented growth of L10FePt on Si substrate. Acta. Phys. Pol. A 127, 611–613 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roghani, R., Sebt, S.A. & Khajehnezhad, A. The texture ordering in L10-FePt–Ag nanocomposites. J Theor Appl Phys 14, 47–51 (2020). https://doi.org/10.1007/s40094-019-00359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-019-00359-z