Abstract
Purpose
The study investigated the effects of poultry and goat manures on the kinetics of potassium fixation and release in some sandy loam and loamy soils of Ogun State, Nigeria.
Methods
The treatments consisted of poultry and goat manures applied at 25 g and 100 g/5 kg soil set in completely randomized design with three replicates. Potassium fixation and release kinetics were computed from the analytical data.
Results
Experimental soils was sandy, slightly acidic, low in nutrients with 80% fixed potassium. However, manure application resulted in 74% reduction of the amount of K fixed by the soils. Elovich and power functions had the best fit for K released in soils treated with goat manure. The K release pattern in poultry manure-amended soil is best described by the parabolic diffusion, Elovich, and power functions, while the first-order equation described K release in soils treated with cattle manure. The potassium release rate constant correlated positively with K uptake.
Conclusion
The ability of the studied soils to fixed K was reduced with the application of organic manures. Potassium fixation decreased with increase in organic manure rates, 100 g/5 kg soil tends to be the optimum rate, and poultry manure had greater effect on the fixing and releasing power of K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potassium (K) fixation is common in most soils, and this underscores the importance of the nutrient addition as fertilizer nutrient and its availability to plants. Fixation of soil K is a function of many soil factors such as the types and amount of soil clay. Omueti and Laukullich (1988) reported that the presence of aluminum (Al) in soil interlayer 2:1 and mixed-layered silicates exhibit a pronounced affinity for K. This may invariably modify K exchange reactions and availability to crops in highly weathered soils of the tropics. However, specific adsorption sites for K in the soils have made the nutrient strongly adsorbed than Al, Ca, or Mg in tropical soils (Tening et al. 1995). As a result of this, cycling of nutrients and the residual effect of K fertilization on the soils, consequently, improve. Majority of the soils in subhumid zone of Nigeria have been observed to have the ability to fix applied K (Tening et al. 1995); however, Adeoye et al. (2008), reported that the K fixing of some of these soils could be ascribed to the soil type. It is established that K released from micaceous clays like muscovite or biotite occurs when there is exchange between the K and hydrated cations; it may also be as a result of mica dissolution which takes place before the formation of weathering products. The importance of these two (muscovite and biotite) in K chemistry is based on the stability of mica and soil environments (Sparks 2000). The presence of large amount of K in the interlayer lattice is determined by the content of K in the soil with respect to the soil critical K level. Several studies (Agyenim-Boateng et al. 2006; Srinivasarao et al. 1999), however, reported that biological activity promotes potassium release in the soil. Such biological activity could be promoted by the addition of organic manures. This is even more important in Nigeria where the soils are mostly deficient in K and large quantities of the animal manures produced are considered as waste products from the livestock industry. Many soils in Nigeria are deficient in K and the status of this element varied widely among soils (Taiwo et al. 2009). For instance, exchangeable K in these soils will contribute to K nutrition in plant under exhaustive cropping without K fertilizer application; K removal from soil and input of non-exchangeable K-to-K uptake is about 90–95% of the total K uptake in plant (Srinivasarao et al. 1994). Crop K nutrition is affected by the clay mineral type and the soil organic matter content. Therefore, for optimum crop growth, external inputs to improve soil K fertility are necessary by the addition of organic or inorganic fertilizers. Organic fertilizers release nutrients into the soil to nourish soil habitats; they release minerals slowly and steadily for plant growth and development (Erin 2007), but the inorganic fertilizer is costlier than organic fertilizers and unavailable to farmers. It produces harmful effects on the soil by increasing the soil acidity and degrading soil properties; on the contrary, the organic fertilizer serves not only as sources of nutrients in plants but also improves soil physical properties (Nottidge et al. 2005). In production of crops, water soluble K and exchangeable K are exhausted by crop uptake (Samadi 2010) and, therefore, need to be replaced continually with K through the release of fixed K. Consequently, it is hypothesized that addition of manures will affect the K chemistry and subsequent release in soils. Hence, this research was designed to study the effect of poultry and goat manures on soil capacity to fix and release added potassium.
Materials and methods
Description of the study area
The research was carried out in May 2016, on six soil samples collected from six different locations in Ogun State, Nigeria. The state has a land area of about 16,726 km2. The state is within the rain forest zone and partly within the southern Guinea savanna region of the Nigeria’s agro-ecological belt (Sodiya et al. 2009). The rainfall pattern is bimodal, thereby making it fit for production of crops like maize, rice, and vegetables. The vegetational cover of the state ranges from mangrove forest in the southeastern part of the state to the diverse forest communities and guinea savanna in the northwest; this also enhances livestock production (Sodiya et al. 2009).
Soil sampling
Surface soil (0–20 cm) samples were collected from Ijale–Papa (7°12′ 00″N:3°12′ 00″E), Aiyetoro (6 42′0″N: 20 49′0‴E), Ipetumodu/Agbadu (8 5′0″N; 33.35′E), Ojere (7 6′ 0″N: 3.43′E), Olorunda (7.21′N; 3.37′), and Ilewo–Orile (7.22′N; 3.43′E); they belong to three local soil series of Apomu, Iwo, and Ibadan series. The soils of the locations are Alfisols, characteristically kaolinitic with some Fe and Al oxides in trace amounts.
Pot experiment
Five kilograms of the sieved soil samples were placed in plastic pots (10 l capacity) with drainage holes at the bottom. Small rates of organic manures were used to get a clear understanding of the fixing abilities of the soils and release of potassium. The poultry and goat manures were air dried before incorporation into pots separately at 25 and 100 g/pot; no manure was added to the control pots. This is equivalent to applying approximately 1.25 and 5 tons manure per hectare. The soil and manure mixture were irrigated to 70% field capacity and left for 14 days for equilibration. Thereafter, the manure-treated soil samples were taken from each pot to investigate the soil K-fixing capacities and release kinetics. The experimental units are arranged in completely randomized design.
Soil analysis
The soils textural classes were determined by the hydrometer method as used by Gee and Bauder (1986). Soil pH was measured using glass electrode attached to a pH meter (HANNA pH meter); this was done in a 1:2.5 soil–water ratio (Page et al. 1982). The exchangeable acidity was extracted with 1 M KCl solution and titrated against 1 M NaOH. Soil organic carbon was determined by dichromate acid oxidation procedure (Nelson and Sommers 1982). Exchangeable bases (K, Ca, Mg, and Na) in the soils were extracted with 1 M NH4OAc, and determined by flame photometry (K and Na), while Ca and Mg were determined by atomic absorption spectrophotometry (Knudsen et al. 1982). Mixtures of HNO3 and H2SO4 (1:1) were used to digest the soil samples for total K. Available K was extracted with 1 M NH4OAc; soil–water suspension (1:5) was used to extract for solution K after shaking for 60 min (Sharpley 1987). Exchangeable K was obtained by deducting solution K from NH4OAc-K. The difference between total K and NH4OAc-K gave non-exchangeable K, while mineral K was deduced from the difference between the non-exchangeable K and the fixed K. Flame photometer (JENWAY) was used to measure all forms of K (Sharpley 1987).
Potassium fixation
Potassium fixing capacities of the soils were investigated using the method of Jackson (1979) as used by (Sahu and Gupta 1987). Exchangeable potassium was extracted with 0.5 M CaCl2 solution; 5 g each of the treated soil was placed in a polypropylene centrifuge tubes with 50 ml of 5, 25, 45, 97, and 120 mg L−1 potassium solution as KCl; 10 ml of distilled water was added. After shaking for 3 h and 72 h’ equilibration, the soil suspension was centrifuged; fixed K was determined in the liquid phase using flame photometer. The reading from flame photometer gave the concentration of fixed K in ppm; this was converted to cmol kg−1 by the expression: ppm/39 × 10. The product of the expression divided by the total K multiply by 100 gave the % fixed K in the studied soils.
Potassium release kinetics
One gram of treated soils was transferred into 50 ml centrifuge tubes containing 10 ml of 0.05 M citric acid and were shaken for 1, 2, 4, 8, 16, 32, 64, and 128 h, at the end of the equilibrium periods, the supernatants were separated by centrifugation, in duplicates, and extraction was repeated by adding another 10 ml citric acid (Simard et al. 1993); K in the supernatant was measured.
Potassium released with time was fitted to the four mathematical models;
-
1.
First-order equation: ln (Ko − Kt) = a − bt (Martin and Spark 1983).
-
2.
Parabolic diffusion equation: Kt/Ko = a + b \( \surd t \).
-
3.
Elovich equation: Kt = a + lnt (Sparks 1989).
-
4.
Power function: lnKt = a + b lnt (Havlin and Westfall 1985).
Potassium released at time (t) = Kt, Ko is the highest content of K released, a and b are constants, and (t) is time in hour. The constants ‘a’ and ‘b’ were calculated from the slope and the intersection with y-axis of the plot lnX vs lnt, respectively. The rate constants (b) were calculated from the slope of the linear regression equation which was fitted (Dimirkou 1994).
Results and discussion
All the studied soils had high sand content (Table 1); such soils cannot hold appreciable amount of water or nutrients for plants, so there will be need to improve the soil’s water and nutrient holding capacity by the addition of organic amendments. The content of quartz in the parent material of the studied soils may be high which probably led to high values of the sand fraction (Brady and Weils 1999). All the studied soil was acidic (pH range of 5.44–6.51) in nature; this may be due to the soil management practices or the sandy texture could have promoted the leaching of soil basic cations. The normal pH values of soils of the region are 5.5–7.5 (Agbogidi and Ejemete 2005). The acidic nature of the studied soils could be a result of the natural removal of the exchangeable bases and separation of strong and functional groups in the organic matter (Esu 2001). The low nutrients observed in the soils (Table 1) may be due to leaching of the soil basic cations; more than 75% of the soil separates are sand and the consequent low soil pH. This agrees with the reports of (Ojeniyi and Akanni 2008); they reported nutrient deficiency in soils of Southwestern part of Nigeria, because they are mostly weathered Alfisols. Rapid decomposition of organic matter by high solar radiation might be responsible for low organic matter concentration. Landor (1991) also reported natural removal of organic materials through continual burning as a major contributor to the reduction in organic matter accumulation in the soil. The low total nitrogen content observed of the soils is related to the low level of organic matter. Soils with low pH have high fixing power for phosphorus resulting in low content of the nutrient in the studied soils (Nnaji et al. 2002). Low values were observed for effective cation exchange capacity and exchangeable acidity in the soil samples; these could be a result of washing out of exchangeable bases in the soil (Olatunji et al. 2007). The low electrical conductivity (0.02–0.96 ds m−1) of the studied soils could be responsible for the low concentrations of Ca2+, Mg2+, and Na+ observed (Warman and Termer 2005; Egbuchua 2007).
Potassium status in studied soils
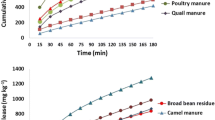
The solution K observed in the soils from Ijale–Papa, Agbadu, Olorunda, and Ilewo–Orile, was lesser than the critical value (0.05 cmol kg−1s) proposed by International Potash Institute (2001), while values observed in soils obtained from Aiyetoro and Ojere were greater than the critical value (Table 2). The solution K consisted 16.67–75% of NH4OAc-K. The total K in the studied soils ranged from 66.61 cmol kg−1 in Ijale–Papa to 113.20 cmol kg−1 in Agbadu. The low content does not retard the release of exchangeable K, but it is not enough to sustain plant growth and is very low compared with the requirement for most crops (Datta and Sastry 1988). The values of NH4OAc-K (0.04–0.34 cmol kg−1) observed were lower than the values of 0.4–2.0 cmol kg−1 reported by Darwish et al. (2003). Considering the critical value of NH4OAc-K (0.41 cmol kg−1) reported by Al-Zubaidi et al. (2008), all the studied soils were low in NH4OAc-K, and hence, the soils are expected to respond positively to potassium fertilization. The study showed that NH4OAc-K constituted a substantial part of K supplying power of the studied soils, because it consisted 28.57–88.57% of fixed K. A large content of the K in the studied soils occurred as fixed K; this signifies that the studied soils are assumed to have high supplying power for long-term cropping (Chen et al. 2001). The clay mineral types in the soil could be responsible for the high K-fixing capacity; this was, however, not verified empirically in the study. Potassium in the six soils was observed to occupy a small portion of cation exchange capacity (CEC) of the soils (Table 2); this was showed by the average % K saturation (8.78%) of the soil samples (Al-Zubaidi et al. 2008). This result was similar to the observations of Eghball and Power (1999) who observed increase in soil K after the application of compost made from swine and chicken manure. Statistical analysis revealed the significant correlations (p < 0.05) between the amount of fixed potassium and cation exchange capacity (CEC) in the studied soils; there was no significant correlations (p > 0.05) between fixed K, % organic matter, % clay, and % silt. Hosseinpur and Safari Sinegani (2007) reported similar relationship between fixed K and cation exchange capacity, but his observation was different from the relationship with % clay, % silt, and % organic matter. They reported a significant correlation between the latter and the soil properties, and the result revealed that the studied soils were characterized by relatively high potential for potassium fixation (Tables 3, 4). The amount of potassium released by successive extractions increased with time; the apparent equilibrium was obtained after 64 h (Fig. 1a–d). The patterns of the curve were related to the initial content of potassium. The curves showed two stages of release, which was characterized by extraction of soluble and weakly bonded potassium (mostly exchangeable K); the latter stage which was observed after 32 h of extraction was characterized by release of strongly bonded potassium (mostly non-exchangeable K), this segment is known to be crucial for the replenishment of the labile soil K. The discontinuity of the slope showed different mechanisms controlling release process; it is likely that the K released by mechanism in the weathered periphery (1–32 h) is distinct from slow K released in the interior site (32–128 h) of the clay minerals (Talibudeen et al. 1978). The cumulative K release plots had a pattern similar to the plots demonstrated by Hosseinpur and Kalbassi (2000). The difference in the amount of K released from the studied soils could be ascribed to the nature of the K-bearing minerals which may include: crystal structure, chemical composition, degree of depletion, layer charge alteration, and soil environment (Al-Zubaidi 2003). The results showed that, after 32 h of extraction, a considerable portion of the non-exchangeable K was remaining in the soils; this is an indication that the unreleased non-exchangeable K will be released and utilized by plant for a long period of time. These observations differ from the report of (Datta 1993); he observed equilibrium in the 8th extraction after 128 h which showed that, after this time of extraction, the soils which he studied still had considerable amount of non-exchangeable K remaining. The release of non-exchangeable K was an exchange reaction when this slow exchange occurs; the replacing ion first enters the unexpanded interlayer without its hydration. Simultaneously, the interlayer will expand upon hydration of these ions allowing fixed or trapped K+ to hydrate and slowly diffuse to exchange sites on outer parts of the clay particle (Hosseinpur et al. 2012).
a Potassium released from Ijale–Oapa, Aiyetoro, and Ipetumodu/Agbadu soils. b Potassium released from Ojere, Olorunda, and Ilewo–Orile soils. c Potassium released from Ijale–Papa,Aiyetoro, and Ipetumodu/Agbadu soils. Asterisk without manure; double asterisk soils treated with poultry manure. d Potassium released from Ojere, Olorunda, and Ilewo–Orile soils
Results of the statistical analysis obtained from the graphs, which were fitted between the models and the experimental data reflected by the Coefficients of determination (R2) and the standard errors of estimates (SE) and other constants. First-order best described K released in soils treated with goat manure (Table 5); this finding is in conformity with the report of Hosseinpur and Kalbassi (2000). The parabolic diffusion, Elovich, and power function equations described most appropriately, the K released in soils treated with poultry manure (Table 6), and this is analogous to the report of (Agyenim-Boateng et al. 2006). The first order, Elovich, and Power function equations which demonstrated the best fit also displayed the high potassium (K)-released rate (b) values.
Conclusion
Result from the study revealed that the potassium fixing capacities of the studied soils were reduced after the application of the three different organic manures, and hence, more K was released. The ability to fix K decreased with increase in organic manure rates, 100 g/5 kg soil tends to have more effect on the fixing abilities of the soils, and poultry manure had greater effect on the fixing and releasing power of K.
References
Adeoye GO, Adeluwa OO, Ojelade M, Sridhar MKC, Makinde EA, Olowoake AA (2008) Comparative evaluation of organo-mineral fertilizer and mineral fertilizer (NPK) on yeld and quality maize (Zea mays(L) moench) Nig. J Soil Sci 18:132–137
Agbogidi OM, Ejemete OR (2005) An assessment of the effects of crude oil pollution on soil properties, germination and growth of Gambaya albida(L). Uniswa Res J Agric Sci Tech 8(2):148–155
Agyenim-Boateng S, Zickerman J, Kornahrens M (2006) Poultry manure effect on growth and yield of Maize. West Afr J Appl Ecol 9:61–70
Al-Zubaidi A (2003) Potassium status in Iraqi soils. In: Johnston AE (ed) Proceedings of the regional workshop: “Potassium and water management in West Asia and North Africa”, held at National Center for Agricultural Research and Technology Transfer, NARCTT, Amman, Jordan, 5-6 November 2001. International Potash Institute, Basel, Switzerland, pp 129–142
Al-Zubaidi A, Yanni S, Bashour I (2008) Potassium status in some Lebanese soils. Lebanese Sci J 9(1):81–95
Brady C, Weils RR (1999) Nature and properties of soil, 12th edn. Prentice Hall, New Delhi, pp 74–114
Chen JH, Wu J-T, Huang W-T (2001) Effect of applied compost or the availability of nitrogen and phosphorus in strongly acidic soils. Chin J Agric Chem Soc 34:112–117
Darwish T, Nasri T, el Moujabber M, Atallah T (2003) Impact of soil nature and mineral composition on the management and availability of potassium in Lebanese soils. In: Johnston AE (ed) Proceedings of the regional workshop: “Potassium and water management in West Asia and North Africa". IPI, pp 152–160
Datta SC (1993) Potassium release in relation to mineralogy of soil and clays. J Ind Soc Soil Sci 41(3):658–662
Datta SC, Sastry TG (1988) Determination of threshold levels for potassium in three soils. J Indian Soil Sci 35:676–681
Dimirkou A (1994) Kinetics of potassium adsorption by Entisols of Greece. Commun Soil Sci Plant Anal 25(9–10):1417–1430. https://doi.org/10.1080/00103629409369124
Egbuchua CN (2007) Pedogenic characterization and evaluation of some wetland soils in Delta. Ph.D Thesis of Delta state University, pp 65–69
Eghball B, Power J (1999) Phosphorus and nitrogen based manure and compost applications: maize production and soil phosphorus. Soil Sci Soc Am J 63:895–901. https://doi.org/10.2136/sssaj1999.634895x
Erin H (2007) “Organic farming” Microsoft Student 2008 (DVD). Microsoft Corporation, 2007, WA. Microsoft Encarta 2008(C) 1993–2007 Microsoft Corporation
Esu IE (2001) Pedological characterization of soils of alluvial complex of Nigeria. Ibadan. Pedological handbook, pp 171–190
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis (part 1) physical and mineralogical methods. Agronomy, monograph 9 (ed). Am Soc Agro/Soil Sci Soc of Am. Madison, pp 12–17:383–411
Havlin JL, Westfall DG (1985) Potassium release kinetics and plant response in calcareous soils. Soil Sci Soc Am J 49:366–370
Hosseinpur AR, Kalbassi M (2000) Potassium quantity intensity ratio of some soils of Iran and correlation of its parameters with selected soil properties (in persian). J Sci Tech Agric Nat Res 4(1):43–57
Hosseinpur AR, Safari Sinegani AA (2007) Soil potassium-release characteristics and the correlation of its parameters with garlic plant indices. Commun Soil Sci Plant Anal 38:107–118. https://doi.org/10.1080/00103620601093751
Hosseinpur AR, Motaghian HR, Salehi MH (2012) Potassium release kinetics and its correlation with pinto bean (Phaseolus vulgaris) plant indices. Plant soil Environ 58(7):328–333
International Potash institute IPI (2001) Potassium dynamics and its availability. International fertilizer Correspondent, pp 1–5
Jackson ML (1979) Soil chemical analysis. Advanced course, 2nd. Published by the author, Madison, Wisc
Knudsen D, Peterson GA, Pratt PF (1982) Lithum, sodium and potassium. In: Page AL et al (eds) methods of soil analysis, part 2.2nd edition. Agronomy 9:403–429
Landor JR (1991) Booker tropical soil manual. A hand book for survey in the tropics and subtropics. Longman Group. England, pp 106–144
Martin HW, Spark DI (1983) Kinetics of non-exchangeable potassium release from two coastal plain soils. Soil Sci Soc Am J 47:883–887. https://doi.org/10.2136/sssaj1983.03615995004700050008x
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL (ed) Methods of soil analysis. Part 2. 2nd, Agronomy 9. American Society of Agronomy, Madison, pp 539–547
Nnaji G, Asadi U, Mbagwu JSC (2002) Evaluation of the physic–chemical properties of soils under selected agricultural land utilization types. J Trop Agric Food Environ Ext 3:27–33
Nottidge DO, Ojeniyi SO, Asawalam DO (2005) Comparative effect of plant residue and NPK fertilizer on nutrient status and yield of maize in a humid Utisol. Nig J Soil Sci 15:1–8
Ojeniyi SO, Akanni DI (2008) Residual effect of goat and poultry manure on soil properties content and yield of amarathius in South West Nigeria. Res J Org Waste Agric 1(11):1–7
Olatunji CA, Ighodo U, Igiri JN (2007) Soil survey report of the Niger Delta. Federal Government Action of the Niger Delta, p 158
Omueti JAI, Laukullich LMI (1988) Identification of clay minerals in soil. The effect of sodium pyrophosphate. Soil Sci Soc of Am J 52(1):285–287. https://doi.org/10.2136/sssaj1988.03615995005200010051x
Page, Miller AL, Keeney DR (1982) Methods of soil analysis, 2nd edn. Am Soc Agronomy Madison WI, pp 55–61
Sahu S, Gupta SK (1987) Fixation and release of potassium in some Alluvial soils. J Indian Soc Soil Sci 35:29–34
Samadi A (2010) Long-term cropping on potassium release and fixation behaviors”. Arch Agron Soil Sci 56(5):499–512. https://doi.org/10.1080/03650340903161195
Sharpley AN (1987) The kinetics of soil potassium desorption. Soil Sci Soc Am J 51:912–917. https://doi.org/10.2136/sssaj1987.03615995005100040015x
Simard RR, Dkimpe CR, Zizka J (1993) Release of potassium and magnesium from soil fractions and its kinetics. Soil Sci Soc Am J 56:1421–1428. https://doi.org/10.2136/sssaj1992.03615995005600050015x
Sodiya CI, Adedire MO, Lawal-Adebowale OA (2009) Land holding rights of Fulani Pastoralists and its effect on their agropastoral production system in Ogun State, Nigeria. Tropicultura 27(2):65–69
Sparks DL (1989) Kinetics of soil chemical processes. Academic, England
Sparks DL (2000) Bioavailability of soil potassium. In: Esumme M (ed) Handbook of soil science. CRC Press, Boca Raton
Srinivasarao CH, Khera MS, SubbaRao A (1994) Soil potassium depletion and K replenishment capacity of soils under intensive cropping. J Potassium Res 10:229–235
Srinivasarao CH, Swarup A, SubbaRao A, Rajagopal V (1999) Kinetics of non-exchangeable potassium release from a Tropaquept as influenced by long term cropping, fertilization and manuring. Austr J Soil Res 37:317–328. https://doi.org/10.1071/S98049
Taiwo AA, Adetunji MT, Azeez JO, Bamgbose T (2009) Potassium supplying capacity of some tropical Alfisols in Southwest Nigeria as measured by intensity, quantity and capacity factors. Nutr Cycl Agro-Eco 86:341–355. https://doi.org/10.1007/s10705-009-9296-1
Talibudeen O, Beasley JD, Leone P, Rajendran N (1978) Assessment of soil potassium reserves available to plant roots. J Soil Sci 29:207–218. https://doi.org/10.1111/j.1365-2389.1978.tb02051.x/full
Tening AS, Omueti JAI, Tarawali G (1995) Fixation of potassium in some soils of the sub-humid zone of Nigeria. Commun Soil Sci Plant Anal 26(7–8):1169–1177. https://doi.org/10.1080/00103629509369362
Warman PR, Termer WC (2005) Evaluation of sewage sludge, septic waste and sludge compost applications to corn and forage: yields and N, P and K content of crops and soils. Biores Tech 96:955–961. https://doi.org/10.1016/j.biortech.2004.08.003
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Taiwo, A.A., Adetunji, M.T., Azeez, J.O. et al. Kinetics of potassium release and fixation in some soils of Ogun State, Southwestern, Nigeria as influenced by organic manure. Int J Recycl Org Waste Agricult 7, 251–259 (2018). https://doi.org/10.1007/s40093-018-0211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-018-0211-0