Abstract
Background
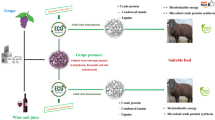
Crop residues and agro-industrial by-products, available in appreciable quantities, can play a significant role in the nutrition of ruminants. The appropriate utilization of by-products in animal nutrition can improve the economy and the efficiency of agricultural, industrial and animal production. The present work investigates the bio-conversion of olive cake (OC), generated by the olive oil industries in Egypt, using locally isolated filamentous fungi in solid state fermentation, so as to upgrade its nutritional values and digestibility for its use as ruminants feed.
Methods
Seven non-mycotoxin producing fungal strains namely Trichoderma reesei F-418, T. harzianum F-416, T. virdie F-520, T. koningii F-322, Aspergillus oryzae FK-923, A. fumigatus F-993, and A. awamori F-524 were cultured on OC for 7 days at 36 °C. Subsequently, the chemical composition and lignocellulolytic enzyme activities of the resultant substrate were determined.
Results
The most promising result was obtained by A. oryzae FK-923, whereas, an increase in crude protein content ranging from 9.5 % (untreated) to 17.4 % (treated) was detected, while phenols were decreased from 3.1 to 0.92 % and fibers declined from 33 to 22.2 %. A reduction in the values of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were reported. The addition of sugar cane molasses at 2 % showed an increase in crude protein to 18.9 % with a reduction in phenols and fibers to 0.69 and 21.8 %, respectively. Furthermore, the addition of active dry yeast (Saccharomyces cerevisiae) at 1.5 % to the fermentation medium raised the crude protein to 20.2 % (w/w), while phenols and fibers were declined to 0.55 and 19.2 %, respectively.
Conclusions
Therefore, the present findings revealed A. oryzae FK-923 to be an efficient organism for lignocellulolytic enzymes production and simultaneous enhancement in crude protein and in vitro digestibility of OC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many developing countries, there is a gap between available and required ruminants feed. The productivity of the animals is very low, although experimental data suggest that their production potential is higher. The greatest constraint to livestock productivity is the shortage of feeds and forages. Therefore, some countries import cereal grains in an effort to increase livestock production and meet the ever-increasing demand for animal products. The Middle East countries raise some 99 million sheep and goats, 104 million beef cattle and 202 million dairy cattle (FAO 1981). Higher livestock productivity, however, should be sought through better use of locally available feed resources. Crop residues and agro-industrial by-products, which are available in appreciable quantities from processing of fruits and vegetables, can play a significant role in the nutrition of ruminants (Economides et al. 1981). In Egypt, although there are about 30 million tons of agricultural by-products produced annually, only 4.15 million tons of crop residues are used for feeding ruminants (Ministry of Agriculture 2006). The main factors limiting the utilization of crop residues in animal ration are the high crude fiber content, low crude protein, low digestibility and poor feed palatability (Abd El-Rahman et al. 2014). The gap between the availability and requirements of animal feeds in Egypt is about nine million tons of dry matter, equivalent to almost four million tons of total digestible nutrients per year (Bendray et al. 2006). Therefore, efforts should be done to efficiently use all the available by-products and wastes to decrease the ruminants feed shortage in Egypt (AboSalim and Bendary 2005).
Olive cake (OC) is the solid residue obtained after olive oil extraction. It is one of the most abundant agro-industrial by-products in the Mediterranean area constituting a source of environmental problems resulting from its accumulation and disposal (Azkar et al. 2008; Hachicha et al. 2008). In Egypt, there are about 118,382 feddan planted with olive trees, the fruitful area 96,810 feddan, produce about 314,450 ton of olive, representing 3.25 ton/feddan (Ministry of Agriculture and Land Reclamation, 2012). Basically, 100 kg of olives produced approximately 35 kg of crude OC. The utilization of OC as ruminants feed is a good way of recycling this by-product. However, the OC contains a large proportion of cell wall constituents, which renders it unpalatable and poorly digestible to the ruminants. Therefore, attempts have been made to improve the nutritive value of OC through different chemical agents, but results have been more or less satisfactory (Rowghani et al. 2008). Apart from the chemicals, the biological treatment is drawing much attention due to its potential advantages over chemical/physical treatments such as greater substrate and reaction specificity, lower energy requirements, lower pollution generation and higher yields of desired products (Misra et al. 2007; Canet et al. 2008).
Solid-state fermentation (SSF) technique using filamentous fungi has been reported to be a relatively low-cost appropriate technology for the upgrading of lignocellulosic materials in animal feeding (Ugwuanyi et al. 2009). Additionally, SSF has proven to be an efficient way to produce lignocellulolytic enzymes since it provides the filamentous fungi with environmental conditions similar to those of their natural habitat. Besides, the restricted water availability reduces the possibilities of contamination by bacteria and yeast (Neifar et al. 2009). It is well documented that the selection of a lignin degrading fungus is preferable for improving the animal feed quality without compromising much the cellulose and hemicellulose constituents. Therefore, the aim of the present study was to apply farm scaling up using biological fungal treatment of OC in SSF using Aspergillus oryzae FK-923 to reduce the anti-nutritional components like phenols and fibers. Besides, an increase in protein and gross energy contents in a short period of time was reported which improved the nutritive value of olive cake for its investment as animal feeds to overcome nutritional Gabe in Egypt. To the best of our knowledge, this is the first report on the nutritional evaluation of SSF olive cake with A. oryzae FK-923.
Materials and methods
Chemicals
All chemicals were purchased from Sigma–Aldrich or Fluka and were certified reagent grade.
Microorganism
Seven non-mycotoxin producing fungal strains namely Trichoderma reesei F-418 (T1), T. harzianum F-416 (T2), T. virdie F-520 (T3), T. koningii F-322 (T4), Aspergillus fumigatus F-993 (A1), A. oryzae FK-923 (A2), A. awamori F-524 (A3) and Saccharomyces cerevisiae F-707, obtained from the culture collection of Microbial Chemistry Department, National Research Centre (NRC), were grown for 5 days on potato dextrose agar slants (PDA) at 28 °C, and maintained at 4 °C. The slants were subcultured routinely every 3–4 weeks interval.
Preparation of spore suspension
Ten mL of sterilized water with 0.1 % Tween-80 solution was added to 5-day-old culture slants and agitated thoroughly using a shaker to suspend the spores. The number of the spore was adjusted to 1 × 106 spores/mL and was used as an inoculum throughout the study.
Screening medium
One mL aliquot (v/v) of inoculum size (1 × 106 spores/mL) was used to inoculate 500 mL Erlenmeyer flasks each containing 50 g of sugar beet pulp (SBP) moistened with distilled water to a moisture level of 65 % (v/w). The inoculated flasks were incubated for 4 days at 32 °C in a static condition. For fungal inoculum propagation, each flask from the above step was transferred to 10 L capacity Erlenmeyer flask contained 1 kg sterilized moistened SBP, and then incubated statically at 32 °C for 4 days.
Preparation of A. oryzae FK-923 inoculum
Three days old slants cultures of A. oryzae FK-923 was crushed into flasks containing 20 mL of sterilized water. The fungal spores suspensions were used at 10 % (v/w) to inoculate 500 mL capacity flasks containing 20 g of the ground waste moistened at a solid: liquid ratio of 1:2 with a basal medium composed of (g/L): sugar cane-molasses, 40; urea, 2.0; K2HPO4, 1.5 and MgSO4·7H2O, 0.5. The inoculated flasks were then incubated at 36 °C for 72 h under static solid state fermentation system. After fermentation period, the above-prepared inocula of A. oryzae FK-923 were employed to inoculate 200 g of wastes under study moistened by the above basal medium at a solid: liquid ratio of 1:2 and packed in polyethylene bags (40 × 60 cm). The inoculated bags were incubated under static conditions for 7 days at 36 °C. At the end of incubation period, such bags were opened and oven dried at 70 °C for chemical analysis.
Scaling up using fungal treatments
The above-fermented bags were employed to inoculate polyethylene bags (150 × 225 cm) containing 10 kg of ground wastes moistened with the basal medium at a solid: liquid ratio of 1:2. The bags were incubated in a 3 × 3 m room maintained at 36 °C for 7 days. After the incubation period, the fermented wastes were air-dried to 12 % moisture then packed and stored until fed to lactating goats.
Agro-industrial by-products
Olive by-product
The olive cake (OC), resulted from a continuous cold process of olive oil production, were packed in polyethylene bags and kept in a freezer at −20 °C. Before drying, olive cake samples were thawed during 24 h under refrigeration conditions at 5 °C. Drying process was carried out at 90 °C in a convective dryer, at Microbial Chemistry Department, National Research Centre. The dehydrated samples were packed and kept in dark until analysis.
Molasses
Molasses were obtained from Egyptian Sugar and Integrated Company El-Hawmdia, Giza, Egypt.
Sugar beet pulp
Sugar beet pulp (SBP), a by-product of beet sugar industry, was obtained from Abokorkas Sugar Factory, El-Minia, Egypt.
Physico-chemical analysis of olive cake
Protein content
Crude protein determination involved the use of routine Kjeldhal nitrogen assay method with a conversion factor of 6.25 (AOAC 1990).
Lipid content
The lipid content was determined by exhaustively extracting samples in a Soxhlet apparatus, using petroleum ether (Merck, p.a.) as a solvent according to the method described in AOAC method no. 920.39. The crude fiber content was estimated by Weende method through an acid/alkaline hydrolysis of insoluble residues in accordance with AOAC (1990). The ash content was estimated by incineration in a muffle furnace (Felisa, FE-341, Jalisco, Mexico) at 550 °C. All methodologies allowed the recommendations of the official methods.
Determination of total phenolic content
Total phenolic content (TPC) was determined colorimetrically using Folin–Ciocalteu reagent (FC) as described by Chuah et al. (2008) with modifications. 40 mL of absolute ethanol was added to 3–5 g dried and finely crushed sample. The mixture was homogenized during 24 h on a magnetic stirrer then filtered through a Whatman filter paper No 1. The filtrate was washed twice with 40 mL of absolute ethanol then evaporated under reduced pressure at 40 °C on a rotary evaporator (Büchi RE 121, Switzerland). The obtained extract was then dissolved in 100 mL of absolute ethanol and kept refrigerated for further analysis. 0.5 mL aliquot of the OC extract was transferred to a glass tube containing 0.5 mL of reactive FC, after 5 min 2 mL of Na2CO3 solution (200 mg/mL) was added. The sample was then mixed thoroughly on a vortex mixer and the reaction proceeded for 15 min at ambient temperature. A total of 10 mL of ultra-pure water was then added and the precipitate formed was removed by centrifugation for 5 min at 4000×g. Finally, absorbance was measured at 725 nm in a spectrophotometer (Spectronic TM 20 Genesys TM131, IL, USA) and compared to a gallic acid (GA) calibration curve. Results were expressed as mg GA 100/g-dry matter. All measurements were conducted in triplicate and the data were expressed as means.
Chemical analysis
Dry matter (DM) loss was determined by calculating the difference between dry weight before and after fermentation and described as a percentage of initial weight. Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were determined by the detergent system method, while acid detergent lignin (ADL) was measured according to the method described by AOAC (1990). Hemicellulose contents were estimated as the difference between NDF and ADF, while cellulose content was the difference between ADF and ADL. Ash was carried out on dried sample at 105 °C by ignition three samples each 50 g in muffle furnace at 800 °C for 5 h, and the residual ash was calculated as a percentage (%) from the dried initial weight according to AOAC (1970).
Enzyme extraction
After incubation period, the enzyme was extracted with sodium citrate buffer (0.05 M, pH 5.0) (10 mL buffer g−1 substrate), by shaking for 1 h at 180 rpm at room temperature. The extract was filtered through Whatman filter paper No. 1 and centrifuged for 20 min at 5000 rpm (Neifar et al. 2009).
Enzymes assay
Filter paper activity
According to the method described by Bai et al. (2012); filter paper activity (FP-ase) was determined photometrically by measuring the increase in absorbance at 540 nm, after 30 min incubation of 1.0 mL of properly diluted enzyme with 50 mg Whatman filter paper No.1 (1 × 1 cm) and 1.0 mL sodium citrate buffer (0.05 M, pH 5.0) at 50 °C. The enzyme reaction was stopped by the addition of 3 mL of 3, 5-dinitrosalicylic acid (DNS) reagent (Miller 1959). One mL of 40 % Rochelle salt solution was added so as to maintain the color. Absorbance was measured at 575 nm and compared with a standard curve using 0.10–1.0 mg of glucose/mL. One unit of enzyme activity is defined as the amount of enzyme which releases 1 µmol of glucose per min, under the standard assay conditions.
Xylanase activity
Xylanase activity was estimated according to Bailey et al. (1992) using 1 % xylan from oat as the substrate. Xylan was dissolved in 0.05 M Glycine NaOH buffer (pH 9.0). The reaction mixture, containing 0.1 mL of an appropriate dilution of the enzyme and 0.5 mL of the substrate, was incubated for 30 min at 37 °C. The reaction was terminated by placing tubes in a boiling water bath for 10 min. The supernatant was analyzed for reducing sugar with DNS reagent and absorbance was read at 575 nm. A standard curve of d-xylose was used as reference. Each unit of xylanase activity (U) was that amount of activity which released 1 μmol d-xylose min−1.
Glucoamylase activity
Glucoamylase activity was determined according to the method reported by Miller (1959), by incubating 1 % (w/v) maltose with 0.9 mL sodium citrate buffer (0.05 M, pH 5.0) and 0.1 mL of diluted enzyme solution at 50 °C for 30 min. The reaction was terminated by placing tubes in a boiling water bath for 10 min. The released reducing sugars were measured with DNS reagent using glucose as a standard. Glucoamylase activity unit (U) was expressed as the amount of enzyme releasing 1 μmol of glucose per min under assay conditions.
Alpha-mylase activity
Alpha-mylase activity was determined in the culture filtrate by measuring the amount of starch hydrolyzed in the reaction mixture by the iodine method (Manning and Campbell 1961). One unit of enzyme activity has been defined as the amount of enzyme that hydrolyses 1 μmol of starch min−1 under assay conditions.
Protease activity
Protease activity was determined as described by Agrawal et al. (2005). 1 mL of suitably diluted enzyme was mixed with 5 mL of (2 %) casein solution (0.05 M Tris–HCl buffer, pH 8.0), and incubated at 40 °C on a gyratory shaker (200 rpm) for 30 min. An aliquot (0.5 mL) of the reaction mixture was withdrawn and the reaction was quenched by adding 1.5 mL pre-chilled trichloroacetic acid (10 %). The reaction tube was immersed in an ice bath (5 min) to completely precipitate the protein. The supernatant was recovered by centrifugation (10,000×g, 10 min). Tyrosine liberated during casein hydrolysis was measured in the supernatant using the method of Lowry et al. (1951). One unit of protease activity was defined as the amount of enzyme liberating 1 μmol of tyrosine per min under the standard assay conditions. The protease activity is reported per gram of dry solids used in the initial extraction.
Phytase activity
Phytase production was measured using a colorimetric method by following the release of inorganic phosphate from phytic acid according to the method presented by Dhiman et al. (2002). For this purpose, 150 μL of enzyme solution were mixed with 600 μL Tris–HCl (0.1 M, pH 7.0) supplemented with 2 mM sodium phytate and 2 mM of CaCl2 and incubated at 37 °C for 30 min. The reaction was terminated by the addition of 750 μL of 5 % trichloroacetic acid, after which 1.5 μL of the color reagent were added to generate phosphomolybdate. The concentration of inorganic orthophosphate (pi) in this mixture was determined colorimetrically by measuring the absorbance of the solution at 700 nm using a Beckman Coulter DU640 Spectrophotometer (Fullerton, CA, USA). The color reagent was freshly prepared by mixing four volumes of 1.5 % (w/v) ammonium molybdate solution supplemented with 5.5 % (v/v) sulfuric acid and one volume of 2.7 % (w/v) ferrous sulfate solution. The results were compared to a standard curve prepared using K2HPO4 as a source of inorganic phosphate at a concentration ranging from 0.0448 to 2.8706 μM. One unit (U) of phytase activity was defined as the concentration of inorganic phosphate, in μmol, released per min under the defined reaction conditions.
Laccase assay
Laccase activity was measured by monitoring the oxidation of 2,2′-azinodi-3-ethyl-benzothiazoline-6-sulfuric acid (ABTS) at 436 nm (Bourbonnais et al. 1998). The nonphenolic dye ABTS is oxidized by laccase to the more stable and preferred state of the cation radical. The concentration of the cation radical responsible for the intense blue-green color can be correlated to the enzyme activity (Majcherczyk et al. 1998). The reaction mixture contained 0.5 mM substrate (ABTS), 2.8 mL sodium acetate buffer (0.1 M, pH 4.5), and 100 μL of suitably diluted enzyme and incubated for 5 min at 30 °C. Absorbance was read at 436 nm in a spectrophotometer against a suitable blank. One unit of enzyme activity was defined as the amount of the laccase that oxidized 1 μmol of ABTS substrate per min under assay conditions.
Polygalacturinase assay
To measure the activity of polygalacturinase, the assay mixture (1 mL) containing an equal volume of enzyme and 1 % (w/v) pectin dissolved in 0.1 M phosphate buffer (pH 7.0) was incubated at 50 °C for 10 min. The reducing sugar released was measured by the dinitrosalicylic acid method. Control samples were prepared with inactivated enzymes. One unit of enzymatic activity (U) was defined as one μmol of galacturoric acid released per min. All the experiments were conducted in triplicate and the data were expressed as a mean. The enzyme activity is expressed in terms of units per gram dry fermented substrate (U/g-ds).
Protein determination
Estimation of total protein was done by using the Bradford method and bovine serum albumin (BSA) as standard (Bradford 1976).
Amino acids analysis
Amino acid analyzer was used for amino acids analysis by hydrolyzing 0.5 g of protein with 10 mL of 6 N HCl under vacuum at 110 °C for 24 h. While, cystine and cysteine were determined after pretreatment with performic acid (Christias et al. 1975).
Results
Chemical analysis of the olive cake
Data presented in Table 1 revealed that, the olive waste has many valid part used in animal and poultry nutrition; it has 9.5 % protein, 5.7 % olive oil, which consider as a good source of energy and antioxidants, and low ash content (7 %). In spite of the previous advantages, there are other components that limit its supplementation in rations by conquerable amounts resample essentially in phenols which characterized by its low biodegradability that eliminate its nutritional value as it inhibits the microflora in the ruminant and the present lignin restricts the utilization of cell plant content by microflora. Besides, the olive cake contains a high crude fiber (33 %) which may be a good carbon source for many microorganisms. So we select different fungal strains known in biological treatments by producing no mycotoxins and applied in food fermentation and used as a probiotic in feeding field.
Effect of biological fungal treatment on the chemical composition of olive cake
OC bio-conversion into a better quality feed by biological treatment in solid state cultures could be an alternative to chemical and physical treatment for enhancing the OC quality for microbial fermentation in rumen. In the current work, it was obvious that the chemical composition of olive cake was affected according to the fungal strain used. Pointed to our aim, A. oryzae FK-923 (A2) gave the most promising results concerning the enhancement of the nutritive value of the OC (Table 2), as the following were achieved according to the importance for nutrition: (1) reduce the phenols to less than one. (2) Increase total protein to 17.4 %. (3) Decrease the crude fibers. (4) Decrease lignin. (5) Increasing gross energy. These results indicated that, the phenolic compounds, which are the more obstacles towards the use of OC by more amounts in rations, was decreased to a limit that did not affect the microflora. Besides, the increased amount of total protein by about 90 kg/ton can be used to substitute other expensive ingredients in feed rations. Moreover, the reduction in crude fibers content makes free to accomplish different feed rations.
Influence of biological fungal treatment on the fermented olive cake recovery
From the data illustrated in Fig. 1, it was clear that the treated OC recovery is correlated to the fungal strain applied. The percent of the recovery obtained with the application of Aspergillus sp. was higher than that with Trichoderma sp., since, A. oryzae FK-923 and A. fumigatus achieved the high recovery percent with 92.2 %.
Effect of biological fungal treatment on the total protein yield of olive cake
Results illustrated in Fig. 2 clearly indicated that, the total protein yield (kg/ton) of the treated OC was varied according to the fungus used and the percent of recovery. Whereas, the highest total protein yield (174 kg/ton) was obtained when A. oryzae FK-923 was used in biological treatment of olive waste, followed by A. awamori F-524 (161 kg/ton). On the other hand, lowest total protein yield was obtained with T. koningii F-322 treatment.
Effect of sugarcane molasses supplementation on the chemical composition of treated olive cake
Form the data presented in Table 3, it was found that the addition of sugar cane molasses has an advantage effect in enhancement of feed quality of OC biologically treated by A. oryzae Fk-923, owing to the increase in protein content. The addition of sugar cane molasses at 2 % (w/w) was more suitable than other tested amounts, as the protein percent raised from 17.4 to 19 % in the fermented olive waste with total protein 166.1 kg/ton. On the other hand, phenols and fibers were reduced to 0.69 and 21.8 %, respectively.
Effect of active dry yeast S. cerevisiae supplementation on treated olive cake
Data presented in Table 4 showed that, the addition of active dry yeast has an advantage in the enhancement of feed quality of OC biologically treated by A. oryzae FK-923, as protein content was increased. On other hand, both phenols and fibers were decreased. Addition of dry yeast at 1.5 kg/ton olive waste was more suitable than other tested amounts, as the protein percent raised to 20.2 % in the fermented olive waste with total protein 170.8 kg/ton. On the other hand, phenols and fibers were reduced to 0.55 and 19.2 %, respectively.
Effect of biological treatment on the chemical composition of olive cake
The fermented OC showed a much lower cell wall component value than that of the untreated OC (Table 5). Whereas, organic matter, crude fiber, nitrogen free extract and total fiber contents in the biological treated olive cake were decreased than that of untreated OC (raw), while crude protein was increased from 9.5 to 20.2 % in the treated OC.
Enzymes involved in fermentation of olive cake by A. oryzae FK-923
The reduction of OC fiber content through the SSF process, using A. oryzae FK-923, is not surprising considering that this fungus possesses an efficient lignino-hemicellulolytic system as shown in Fig. 3. Since livestock and poultry cannot produce sufficient hydrolases enzymes in alimentary tracts, the presence of such enzymes add high nutritive value as well as save adding exogenous enzymes to feeds, thus increasing feed intake and production performance of livestock and poultry; increasing digestibility and absorptive of nutrients; enhancing the digestibility and utilization efficiency of feeds, and thus reducing feed costs.
The amino acid composition of microbial compared to alfalfa protein
The data presented in Table 6 showed that the amino acid profiles of A. oryzae FK-923 culture grown on olive cake have advantage in lysine, arginine, glutamic acid, proline, alanine, methionine and less in cysteine when compared with amino acid composition of alfalfa protein.
Discussion
In Egypt, large quantities of agriculture and agro-industrial by-products are generated and most of them are regarded as waste and non-conventional feedstuffs. The majority of researchers suggest that these lignocellulosic substrates when used as a source of protein, they are equivalent to soybean meal and other sources of protein for beef cattle diets (National Research Council (NRC) 2001; Loy and Wright 2003). The crude OC, used in this study is characterized by low protein content (9.5 %) and relatively high fat content (5.7 %). The OC is rich in fiber with NDF and ADF values of 62 and 48 %, respectively (Table 1). Since OC is rich in fiber, it has a low degradability in the rumen. OC bio-conversion into a better quality feed by A. oryzae FK-923 in SSF could be an alternative to chemical and physical treatment for enhancing the OC quality for microbial fermentation in rumen. The reduction in OC phenolic contents during SSF (Table 2) is an important aspect in the adaptation of A. oryzae FK-923, since the capacity of a substrate to resist to degradation has been partially attributed to its array of phenolic compounds (Fermor and Macauley 1991). These results were in agreement with Salmones et al. (2005) who reported a significant negative relationship between phenolic concentration in coffee pulp and laccase activity.
An increase in protein content from 9.5 to 17.4 % was observed on OC fungal treatment with A. oryzae FK-923 over the untreated sample (Table 2). This result is consistent with the findings of Iyayi and Aderolu (2004) that showed an increase in CP contents Trichoderma viride treated agro-wastes. In addition, Dhanda et al. (1994) reported that the CP content of spent straw was increased from 3.42 to 6.19 % after biological treatment. The CP increase could be the result of increased fungal biomass (Chen et al. 1995) suggesting that the treated substrate is a good protein source for livestock. The improvement of CP contents could also be due to secretion of certain extracellular enzymes into the waste during their breakdown and its subsequent metabolism (Kinfemi et al. 2009). The addition of sugar cane molasses at 2 % (w/w) showed an increase in crude protein to 18.9 % with a reduction in phenols and fibers to 0.69 and 21.8 %, respectively (Table 3). Furthermore, S. cerevisiae supplementation at 1.5 % (w/w) to the fermentation medium raised the crude protein to 20.2 % (w/w), while phenols and fibers were declined to 0.55 and 19.2 %, respectively (Table 4). S. cerevisiae possesses several properties, such as its resistance to high sugar and alcohol concentrations and a high growth rate at increasing temperatures (Dhiman et al. 2002; Jung et al. 2008).
In most bioconversion studies, compositional changes, i.e. neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) followed by estimation of digestibility have been carried out to estimate the nutritive value of the final fermented feed (Shrivastava et al. 2012). In the present study, the fermented OC showed a much lower cell wall component value than that of the untreated OC, due to the decrease of NDF and ADF contents by 44.6 and 32.6 %, respectively (Table 5). These results might be due to the breakdown of lignocellulosic bonds by the fungal enzymes (Bilal 2008). The reduction in the total fibers content from 33 to 19.2 % confirms and proves the ability of the selected fungal strain in producing hemicellulases, cellulases, and polygalacturinase enzymes. In this concern Baraghit et al. (2009) reported that the biological treatments of different crop residues with various fungal and bacteria strains decreased cell wall total fibers content.
The present study revealed that, the phenolic compounds which are the more obstacles towards the use of OC decreased to a limit that not affected the microflora and this confirmed that the fungus could produce laccases under the culture conditions. Laccases (EC 1.10.3.2; benzenediol: oxygen oxidoreductases) are multicopper enzymes belonging to the group of blue oxidases that catalyses oxidation of a wide variety of organic and inorganic compounds, including diphenols, polyphenols, diamines and aromatic amines. One electron at a time is removed from the substrate, and molecular oxygen is used as the electron acceptor (Gianfreda et al. 1999). Some fungal laccases degrade toxic fungal metabolites, such as aflatoxin B1 (Alberts et al. 2009) and are also useful in the field of food microbiology. Furthermore, the chemical analysis indicated that, the gross energy was increased from 4200 to 4320 kcal/kg DM.
The degradation of polysaccharide compounds was associated with the increase in reducing sugars. Enhancing the concentration of these sugars is an additional advantage besides the reduction of lignocelluloses in the improvement of biomass quality through biological treatment. Enzymes assay showed that the residual fermented substrate loaded with glucoamylase 756, alpha-amylase 422.2, xylanase 664.4, celullases (FP-ase) 198.2 and polygalagturinase 76.8 U/g-ds (Fig. 3). Since livestock and poultry can’t produce sufficient xylanase and cellulases in alimentary tracts, the presence of such enzymes add high nutritive value as well as save adding exogenous xylanase and cellulases to ruminants feed, thus increasing feed intake and production performance of livestock and poultry; increasing digestibility and absorptive of nutrients; enhancing the digestibility and utilization efficiency of feeds, and thus reducing feed costs (Yang and Wyman 2004).
Cellulases and xylanases have been used in the feed of mono gastric animals to hydrolyze non starch polysaccharides such as β-glucans and arabinoxylans. Cellulases, used as feed additives alone or with proteases, can significantly improve the quality of pork meat (Kumar and Wyman 2009). Glucanases and xylanases reduce viscosity of high fiber rye- and barley-based feeds in poultry and pig. These enzymes can also cause weight gain in chickens and piglets by improving digestion and absorption of feed materials (Taniguchi et al. 2005; Shrivastava et al. 2011). The detection of phytase in the fermented olive waste may be due to the addition of S. cerevisiae. Phytase (myoinositol hexakisphosphate phosphohydrolase, EC 3.1.3.8) catalyzes the hydrolysis of phytate to myoinositol pentakisphosphate and orthophosphate. Improving the digestibility of proteins and increasing the availability of phosphorus and other minerals, which are usually chelated by phytic acid (Konietzny and Greiner 2002) diminishes the anti-nutritive properties of phytate and prevents environmental pollution. Phytases have been studied in different yeast strains; an extracellular acid phytase was recently purified and characterized from S. cerevisiae by Jin-In et al. (2009).
Therefore, it can be concluded from this study that, untreated olive cake is poor in crude protein but rich in NDF and ADF. The biodegradation of OC using A. oryzae FK-923 resulted in an increase of crude protein and a decrease of fiber fractions and thus an increased digestibility of the resultant substrate. This suggests that SSF–A. oryzae FK-923 system is suitable for conversion of solid waste from olive processing industry into a value added animal feed. However, further research work in these areas is necessary to verify the effectiveness of this degraded substrate on the performance of live animals.
References
Abd El-Rahman HH, Abedo AA, El-Nomeary YAA, Abdel-Magid SS, Mohamed MI (2014) Effect of biological treatments of rice straw on growth performance, digestion and economical efficiency for growing calves. Glob Vet 13(1):47–54
AboSalim IA, Bendary MM (2005) Forage supplies in Egypt resources-maximizing its utilization. In: Proceedings 2nd conference of Animal Product Research Institute Sakha
Agrawal D, Partidar P, Banerjee TPS (2005) Alkaline protease production by a soil isolate of Beauveria feline under SSF condition: parameter optimization and application to soy protein hydrolysis. Process Biochem 40:1131–1136
Alberts JF, Gelderblom WCA, Botha A, Zyl WHV (2009) Degradation of aflatoxin B1 by fungal laccase enzymes. Int J Food Microbiol 135(1):47–52
AOAC (1970) Official methods of analysis. In: William H (ed) Association of Official Analytical Chemists, 11th edn. vol. 1, AOAC, Washington, DC
AOAC (1990) Official methods of analysis. In: Helrich K (ed) Association of Official Analytical Chemists, 15th edn. AOAC, Arlington, Virgina
Azkar T, Tosun I, Kaynak Z, Ozkara E, Yeni O, Sahin E (2008) An attractive agro-industrial by-product in environmental cleanup. J Hazard Mater 33:125–137
Bai S, Kumar MR, Kumar DJM, Balashanmugam P, Kumaran MDB, Kalaichelvan PT (2012) Cellulase production by Bacillus subtilis isolated from cow dung. Arch Appl Sci Res 4:269–279
Bailey MJ, Biely P, Poutanen K (1992) Inter laboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Baraghit GA, Ahmed BM, El-Mahy MA (2009) Digestibility, nutritive value and rumen fermentation of rice straw and sugar cane bagasse treated with a commercial bacterial culture. Egypt J Nutr Feed 12(3):511–522
Bendray MM, Ghanem GHA, Gaafar HMA (2006) Utilization of rice straw for feeding ruminants: (productive performance of lactating buffaloes fed rice straw silage). J Agric Sci Mansoura Univ 31(8):5025–5038
Bilal MO (2008) Effect of molasses and corn as silage additives on cell wall fractions of Mott grass silage with different fermentation periods. J Anim Plant Sci 18(4):102–106
Bourbonnais R, Leech D, Paice MG (1998) Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim Biophys Acta 1379(3):381–390
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Canet R, Pomares B, Cabot B, Chaves C, Ferrer E, Ribó M (2008) Composting olive mill pomace and other residues from rural southeastern Spain. Waste Manag 28:2585–2592
Chen C, Fales SL, Varga GA, Royce DJ (1995) Biodegradation of cell wall components of maize stover colonized by white-rot fungi and resulting impact on in vitro digestibility. J Sci Food Agric 68:91–98
Christias C, Couvarakim C, Georgopoulos SG, Macris B, Vomvoyanai V (1975) Protein content and amino acid composition of certain fungi evaluated for microbial protein production. Appl Env Microbiol 29:250–254
Chuah AM, Lee YC, Yamaguchi T, Takamura H, Yin LJ, Matoba T (2008) Effect of cooking on the antioxidant properties of coloured peppers. Food Chem 111:20–28
Dhanda S, Kakkar VK, Garcha HS, Makkar GS (1994) Biological treatments of baddy straw and its evaluation through ruminant feeding. Indian J Anim Nutr 11(2):73–79
Dhiman TR, Zaman MS, Gimenez RR, Walters JL, Treacher R (2002) Performance of dairy cows fed forage treated with fibrolytic enzymes prior to feeding. Animal Feed Sci Technol 101:115–125
Economides S, Hadjipanayiotou M, Georghiades E (1981) The nutritive value of straw, and barley and lucenna hay, and the nitrogen supplementation on the nutritive value of straw to sheep. Technical bulletin 39, Agricultural Research Institute, Nicosia
FAO (1981) Food loss prevention in perishable crops. FAO Agricultural Service Bulletin, No. 43, FAO Statistics Division, Rome
Fermor TR, Macauley BJ (1991) Microbiology factor contributing to selectivity and nutritional quality of mushroom compost. In: Nair NGT (ed) Proceedings of AMGA/ISMS International workshop-seminar on Agaricus compost. AMGA Limited, Sidney, pp 26–45
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediation J 3(1):1–25
Hachicha S, Sallemi F, Medhioub K, Hachicha R, Ammar E (2008) Quality assessment of composts prepared with olive mill waste water and agricultural wastes. Waste Manag 28:2593–2603
Iyayi EA, Aderolu ZA (2004) Enhancement of the feeding value of some agro-industrial by products for laying hens after their solid state fermentation with Trichoderma viride. Afr J Biotechnol 3:182–185
Jin-In M, Won-Seo S, Kim DC, Soon-Oh N (2009) Purification and biochemical properties of an extracellular acid phytase produced by Saccharomyces cerevisiae CY strain. Process Biochem 44(1):122–126
Jung HK, Park CD, Bae DH, Hong JH (2008) Isolation of alcohol-tolerant amylolytic Saccharomyces cerevisiae and its application to alcohol fermentation. Food Sci Biotechnol 17(6):1160–1164
Kinfemi AA, Mohamed MI, Ayoade JA (2009) Biodegradation of cowpea shells by Pleurotus specie for its use as ruminant feed. World J Agric Sci 5:639–645
Konietzny U, Greiner R (2002) Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int J Food Sci Technol 37(7):791–812
Kumar R, Wyman CE (2009) Effect of additives on the digestibility of corn stover solids following pretreatment by leading technologies. Biotechnol Bioeng 102(6):1544–1557
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J biol Chem 193:265–275
Loy DD, Wright KN (2003) Nutritional properties and feeding value of corn and its by-products. In: White PJ, Johnson LA (eds) Corn chemistry and technology. American Association of Cereal Chemists, Inc, St Paul, pp 571–603
Majcherczyk A, Johannes C, Hüttermann A (1998) Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzym Microb Technol 22(5):335–341
Manning BG, Campbell LL (1961) Thermostable α-amylase of Bacillus philus. J Biol Chem 236:2952–2955
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 3:426–429
Ministry of Agriculture (2006) Economic affairs, sector of agricultural statistics, Egypt. Ministry of Agriculture, Egypt
Ministry of Agriculture and Land Reclamation (2012) Economic affairs, sector of agricultural statistics, Egypt. Ministry of Agriculture, Egypt
Misra AK, Mishra AS, Tripathi MK, Prasad R, Vaithiyanathan S, Jakhmola RC (2007) Optimization of solid state fermentation of mustard (Brassica campestris) straw for production of animal feed by white rot fungi (Ganoderma lucidum). Asian Aust J Anim Sci 20:208–213
National Research Council (NRC) (2001) Nutrient requirements of dairy cattle, 7th rev edn. National Academyof Science, Washington, DC
Neifar M, Jaouani A, Ghorbel RE, Chaabouni SE, Penninckx MJ (2009) Effect of culturing processes and copper addition on laccase production by the white-rot fungus Fomes fomentarius MUCL 35117. Lett Appl Microbiol 49:73–78
Rowghani E, Zamiri MJ, Seradj AR (2008) The chemical composition, rumen degradability, in vitro gas production, energy content and digestibility of olive cake ensiled with additives. Iran J Vet Res 9:213–221
Salmones D, Mata G, Waliszewski KN (2005) Comparative culturing of Pleurotus sp. on coffee pulp and wheat straw: biomass production and substrate biodegradation. Bioresour Technol 96:537–544
Shrivastava B, Thakur S, Khasa YP, Gupte A, Puniya AK, Kuhad RC (2011) White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 22(4):823–831
Shrivastava B, Nandal P, Sharma A, Jain KK, Khasa YP, Das TK, Mani V, Kewalramani NJ, Kundu SS, Kuhad RC (2012) Solid state bioconversion of wheat straw into digestible and nutritive ruminant feed by Ganoderma sp. rckk02. Bioresour Technol 107:347–351
Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T (2005) Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng 100(6):637–643
Ugwuanyi JO, McNeil B, Harvey LM (2009) Production of protein-enriched feed using agro-industrial residues as substrates. In: Pandey A, Singh-Nee Nigam P (eds) Biotechnology for agro-industrial residues utilization. Springer, The Netherlands, pp 77–103
Yang B, Wyman CE (2004) Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol Bioeng 86(1):88–95
Acknowledgments
The authors gratefully acknowledge the National Research Centre (NRC), 33 El-Bohouth St., Giza, Egypt, for the facilities and financial support that enable the authors to accomplish this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fadel, M., El-Ghonemy, D.H. Biological fungal treatment of olive cake for better utilization in ruminants nutrition in Egypt. Int J Recycl Org Waste Agricult 4, 261–271 (2015). https://doi.org/10.1007/s40093-015-0105-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40093-015-0105-3