Abstract
Background
The inhibitive effect of the aqueous extract of Rothmannia longiflora (RL) on the corrosion of mild steel in 1 M HCl and 0.5 M H2SO4 solutions was investigated by weight loss measurement as well as potentiodynamic polarization and electrochemical impedance spectroscopy measurements.
Result
The extract was found to efficiently inhibit the corrosion process in both environments and inhibition efficiency increased with increasing extract concentration as well as rise in temperature. Data from electrochemical measurements suggest that the extract functioned by adsorption of the organic matter on the metal/corrodent interface, inhibiting both the anodic and cathodic half reactions of the corrosion process.
Conclusion
The result obtained from this study clearly shows that RL functioned as a good inhibitor for the corrosion of mild steel in 1 M HCl and 0.5 M H2SO4 solutions and the inhibiting efficiency value increased with the inhibitor concentration. The adsorption of the RL on the mild steel surface in acid solution obeyed Langmuir adsorption isotherm.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Background
Several researchers [1–9] have studied the effects of some synthetic organic inhibitors on the corrosion of steel in hydrochloric and sulphuric acid solutions. The efficiency of these inhibitors is related to the presence of polar functions with S, O or N atoms in the molecule, heterocyclic compounds and π-electrons [10–12]. Such compounds can adsorb onto the metal surface and block the active surface sites, thus reducing the corrosion rate. The polar function is usually regarded as the reaction center for the establishment of the adsorption process [13, 14]. The known hazardous effects of most synthetic organic inhibitors and restrictive environmental regulations [15] have made it necessary to search for cheaper, non-toxic and environmentally friendly natural products as corrosion inhibitors. These natural organic compounds are either synthesized or extracted from aromatic herbs, spices and medicinal plants. Most of the natural products of plant origin are non-toxic, biodegradable and readily available in sufficient quantity in addition to meeting the structural considerations. Various plant parts: seeds, fruits, leave and flowers [16–26] were extracted and used as corrosion inhibitors. The results from these studies confirm that biomass extracts possess remarkable abilities to inhibit the corrosion reaction.
Rothmannia longiflora (RL) belongs to family Rubiaceae and widely distributed in Africa from Gambia east to Sudan and Kenya, and south to Tanzania and Angola. The fruits of RL are commonly used to make blue–black markings on the hands, face and body, sometimes to imitate tattooing. RL is considered to have febrifugal and analgesic properties, and a decoction of the leaves, twigs, bark and roots is applied internally or externally in lotions, washes and baths. In Nigeria, the leaves are used to treat itching skin diseases and the fruit pulp is said to be emetic. No specific chemical study of the colorants or dye-precursors present in the flowers, fruits or leaves is available for RL. From the fruits, branches and leaves however, 4-oxonicotinamide-1-(1-β-d-ribofuranoside) (RBF) has been isolated [27, 28]. The fruit also contains monomethyl fumarate (MMF) and d-mannitol (DMT).
RL has never been studied for the purpose of corrosion inhibition. The present paper reports on the inhibiting effect of leaf extract of RL on the acid corrosion of mild steel. It is believed that the strong adsorption properties of the plants’ pigments on the human skin will be also applicable on the metal surface. Corrosion inhibition efficiency (IE) has been experimentally evaluated using gravimetric, electrochemical impedance and potentiodynamic polarization techniques. In addition, adsorption mechanism and relationship between molecular structure and IE are examined by quantum-chemical calculations.
Methods
Gravimetric measurements
Gravimetric experiments were conducted on test coupons of dimension 3 cm × 3 cm × 0.14 cm. Before each experiment the coupons were abraded using emery papers (grades 200–1000) washed with distilled water and dried in acetone and warm air. The coupons were then weighed and suspended in beakers containing the test solutions using glass hooks and rods. Tests were conducted under total immersion conditions in 300 mL of the aerated and unstirred test solutions. The coupons were retrieved after 24 h, immersed in 20 % NaOH solution containing 200 g L−1 of zinc dust, scrubbed with bristle brush under running water, dried and reweighed. The weight loss was taken as the difference between the initial and final weights of the coupons. All tests were run in triplicate and the data showed good reproducibility with standard deviation ranging from 0 to 0.00065. Average values for each experiment were obtained and used in subsequent calculations. The weight losses were used to calculate the corrosion rates as follows:
where Δw is weight loss (mg), s is area of specimen (cm2) and t is the immersion time in h.
The degree of surface coverage (θ) and inhibition efficiency (IE%) were calculated as follows:
w o and w 1 are the weight loss in absence and presence of inhibitor, respectively.
Electrochemical measurements
Metal samples for electrochemical experiments were of dimensions 1.5 cm × 1.5 cm. These were subsequently sealed with epoxy resin in such a way that only one square surface of area 1.0 cm2 was left uncovered. The exposed surface was degreased in acetone, rinsed with distilled water and dried in warm air. Electrochemical experiments were conducted in a conventional three-electrode cell using a VERSASTAT 400 complete DC voltammetry and corrosion system, with V3 Studio software. A platinum foil was used as counter electrode and a saturated calomel electrode as reference electrode. The latter was connected via a Luggin’s capillary. Measurements were performed in aerated and unstirred solutions at the end of 1 h of immersion at 303 K. Impedance measurements were made at corrosion potentials (E corr) over a frequency range of 100 kHz to 10 mHz, with a signal amplitude perturbation of 5 mV. Potentiodynamic polarization studies were carried out in the potential range ±350 mV versus corrosion potential at a scan rate of 0.33 mV s−1. Each test was run in triplicate to verify the reproducibility of the data. The IE% values were obtained from the following equations:
where (R ct,bl) and (i corr,bl) are the polarization resistance and corrosion current density, respectively, measured in solution without inhibitor and (R ct,inh) and (i corr,inh) are the same parameters determined in the solutions containing inhibitor.
Theoretical study
For theoretical study complete geometry of RL molecules was optimized at the DFT Dmol3 module, using DND basis set and the Perdew–Wang local correlation density functional available in Material Studio 4.0 program. The molecular structures were first subjected to geometry optimization using COMPASS force field and then distribution of frontier molecular orbital including the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) obtained from this optimized structure. Although the experimental work here is done entirely in liquid phase, however, based on similar research work [1, 29–31] which has employed gas phase theoretical analysis to evaluate the liquid phase experimental results (they obtained satisfactory correlations between gas phase theoretical analysis and liquid phase experimental results), similar theoretical approach is followed.
Results and discussion
Effect of inhibitor concentration
To study the effect of inhibitor concentration on the IE, weight loss experiments were carried out in both acid solutions at 303 K for 24 h immersion period. The variation of IE with increase in inhibitor concentration is shown in Fig. 1. The extract can be seen to exhibit corrosion inhibiting effects in both acid media, at all concentration used in this study, reaching a maximum IE of 90.92 and 96.50 % in 1.0 M HCl and 0.5 M H2SO4 solutions at the optimum concentration of 1000 mg/L. The values of the weight loss and percentage IE (IE%) obtained from weight loss method at different concentrations of RL leaves at 303 K are summarized in Table 1. The results clearly depict higher corrosion susceptibility of the mild steel specimen in 0.5 M H2SO4 solution as well as a better performance of the extract than in the HCl solution.
Electrochemical impedance spectroscopy (EIS)
In order to obtain information about the kinetics of iron corrosion in presence of RL extracts, the electrochemical process taking place at the open-circuit potential was examined by EIS. EIS measurements of the steel electrode in 1.0 M HCl and 0.5 M H2SO4 solutions without and with the lowest and the highest concentrations of RL were obtained after 30 min at its open-circuit potential. The recorded EIS spectrum for steel in 1 M HCl and 0.5 M H2SO4 is shown in Fig. 2. The impedance diagrams in the absence and presence of the extract show only one depressed capacitive loop, which is attributed to the one time constant. The diameter of Nyquist plots increased on increasing the concentration of RLE indicating strengthening of inhibitive film. The obtained impedance diagram (Nyquist) contains a depressed semicircle and such behavior characteristic of solid electrode is attributed to surface roughness and inhomogeneities of metal electrodes [13, 15, 32]. In these cases, the parallel network polarization resistance and double layer capacitance (R ct–C dl) are usually a poor approximation especially for system where an efficient inhibitor is used. For the description of a frequency independent phase shift between an applied alternating potential and its current response, a constant phase element (CPE) is used instead of capacitance (C). The CPE is defined by the mathematical expression [24, 33, 34].
where Z CPE is the impedance of CPE, A is the CPE constant, ω is the angular frequency, j is the imaginary unit (−1)1/2, n is the surface irregularity. The CPE, which is considered a surface irregularity of the electrode, causes a greater depression in Nyquist semicircle diagram [35], where the metal–solution interface acts as a capacitor with irregular surface. If the electrode surface is homogeneous and plane, the exponential value (n) becomes equal to 1 and the metal–solution interface acts as a capacitor with regular surface, i.e., when n = 1, A = capacitance. The capacitance values of the electrical double layer were calculated according to the following equation [35]:
Simulation of Nyquist plots with Randle’s model containing CPE instead of capacitance and charge transfer resistance (R ct) (Fig. 3), showed excellent agreement with experimental data. The main parameters deduced from the analysis of Nyquist diagram for 1 M HCl and 0.5 M H2SO4 without and containing RL extract are given in Table 2. On increasing RLE concentration from 50 to 1000 mg/L, the charge transfer resistance (R ct) increased and capacitance (A) decreased indicating that increasing RLE concentration decreased corrosion rate. Decrease in the capacitance was caused by reduction in local dielectric constant and/or by increase in the thickness of the electrical double layer. This fact suggests that the inhibitor molecules acted by adsorption at the metal/solution interface [21, 36, 37]. On the other hand the decrease in C dl with increase in concentration was the result of an increase in the surface coverage by the inhibitor, which led to an increase in the IE. The thickness of the protective layer, δ org, was related to C dl by the following equation:
where ε o is the dielectric constant and ε r is the relative dielectric constant. Since adsorption of an organic inhibitor on a metal surface involves the replacement of water molecules and other ions originally adsorbed on the surface, the smaller dielectric constant of organic compounds compared to water as well as the increased thickness of the double layer due to inhibitor adsorption act simultaneously to reduce the interfacial capacitance [38–40]. This provides experimental evidence of adsorption of the extract organic matter on the corroding mild steel surface.
Potentiodynamic polarization measurements
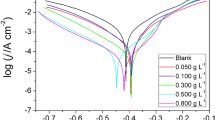
Potentiodynamic polarization plots illustrating the effect of RLE on the anodic and cathodic processes for mild steel corrosion in 1 M HCl and 0.5 M H2SO4 solutions are shown in Fig. 4a, b. The plots illustrate clearly that RLE functioned by different mechanisms in 1 M HCl and 0.5 M H2SO4 solutions. RL functioned via a mixed inhibition mechanism in 1 M HCl solution, reducing the anodic and cathodic currents, with a slight shift of E corr in the anodic direction. In 0.5 M H2SO4 solutions on the other hand, a considerable displacement of E corr in the cathodic direction is observed and the cathodic reaction is significantly inhibited, which means that RL performed essentially as a cathodic inhibitor.
The extrapolation of the Tafel straight lines allows the calculation of the corrosion current density (i corr). The values of i corr, the corrosion potential (E corr), cathodic and anodic Tafel slopes (β c and β a) and IE% are given in Table 3. The IE % was calculated using Eq. (5). The change in β c and β a values as shown in Table 3 indicates that adsorption of RLE modified the mechanism of anodic dissolution as well as cathodic hydrogen evolution. The displayed data show that the addition of RL extract decreased the corrosion current density in both acid media and the decrease was more pronounced with increasing RL concentration. The data obtained from PDP are in good agreement with those obtained from EIS and weight loss methods. Table 4 compares the IE% obtained from the various techniques and shows that the weight loss measurements were higher than those obtained based on the other techniques. This indicates that the adsorption of RL extract is not strongly dependent on the potential in contrast to the results obtained through the polarization resistance. On the other hand, the high IE% values found indicate a strong adsorption favored by the long duration of the weight loss experiment. Therefore, the weight loss experiments confirm the electrochemical results regarding the adsorption of RL extract on the mild steel surface forming a protective film barrier.
Effect of immersion time
The variation of IE with immersion time in both HCl and H2SO4 solutions obtained from weight loss data is shown in Fig. 5. The plot clearly shows a more significant increase in IE% with time in HCl solution compared to the H2SO4 solution. The plot also shows a more rapid decrease in efficiency in the HCl solution suggesting that the extract is more stable in the H2SO4 solution. It obvious that the weight loss varied linearly with immersion time in plain acid and inhibited acid, showing the absence of insoluble product on steel surface [25].
Effect of temperature
Generally, the corrosion rates of mild steel in acidic solutions increase with rise in temperature. This is due to a decrease in the hydrogen evolution overpotential, resulting in higher dissolution rates of metals. A higher rate of hydrogen gas generation increasingly agitates the metal–corrodent interface and depending on the nature of the metal/inhibitor interactions, could hinder inhibitor adsorption, perturb already adsorbed inhibitor or actually enhance inhibitor adsorption. In order to understudy the temperature dependence of corrosion rates in uninhibited and inhibited solutions, gravimetric measurement was carried out in the temperature range 303–333 K in the absence and presence of 50 and 1000 mg L−1 RL. Calculated values of the corrosion rates and IEs are shown in Table 4. Inspection of the data shows that the corrosion rate of the mild steel in all systems increased with temperature, though the effect is significantly subdued in inhibited solution, which means that the rate of adsorption of RL extract increased as well with rise in temperature. Enhanced inhibitor adsorption at higher temperatures is more clearly reflected by the trend of increasing IE with rise in temperature, which is an indication that some of the extract components become well adsorbed at higher temperature and so contribute more to the overall inhibiting effect.
The dependence of corrosion rate on temperature can be expressed by the Arrhenius equation:
where E a is the apparent effective activation energy, R is the general gas constant and A is the Arrhenius pre-exponential factor. A plot of log of corrosion rate versus 1/T gave a straight line as shown in Fig. 6 with a slope of −E a/2.303R.
The values of the activation energies are listed in Table 5. The data shows that the thermodynamic activation functions (E a) of the corrosion reaction of mild steel in 1 M HCl and 0.5 M H2SO4 solutions in the presence of extract are lower than those in the free acid solution indicating that the extract exhibit high IE (%) at elevated temperatures. This means that the extract is more effective at higher temperature. According to Popova et al. [41], unchanged or lowered activation energies in inhibited solutions when compared to that in its absence indicates a specific type of adsorption of the inhibitors, while Szauer and Brandt [42] associate this behavior with the chemisorptions of inhibitor to the metal surface. Taken into consideration the cited references and the E a value calculated from Arrhenius plots, the action of RL extract as a corrosion inhibitor for mild steel in acidic solution can be attributed to strong adsorption bond of a chemisorptive nature.
An alternative formulation of Arrhenius equation is [43, 44]:
where h is the Plank’s constant, N is Avogadro’s number, S* is the entropy of activation and H* is the enthalpy of activation. A plot of log(C R/T) versus 1/T gave a straight line with a slope of −H*/2.303R and an intercept of log(R/Nh) + S*/2.303R, from which the values of S* and H* were calculated and listed in Table 6. The positive signs of enthalpies (H*) obtained in both systems reflects the endothermic nature of dissolution process. The shift towards negative value of entropies (S*) at the optimum concentration imply that the activated complex in the rate determining step represents association rather than dissociation, meaning that disordering decreases on going from reactants to the activated complex [45].
An estimate of the heat of adsorption (Q ads) was obtained from the trend of surface coverage with temperature as follows [46–48]:
where θ 1 and θ 2 are the degree of surface coverage at temperatures T 1 and T 2. The calculated values of both parameters are given in Table 5. The positive values of heats of adsorption (Q ads) indicate that the degree of surface coverage increased with rise in temperature again suggesting that the effectiveness of RL with rise in temperature [46–50].
Adsorption consideration
The mechanism of corrosion inhibition may be explained on basis of adsorption behavior [37, 41]. Basic information on the interaction between the inhibitor and the mild steel surface can be provided by the adsorption isotherm. For this purpose, the values of the surface coverage (θ) at different concentrations of RL in both acid media in the temperature range 303–333 K was used to explain the best isotherm to determine the adsorption process.
The adsorption of inhibitor molecules on the metal surface is a substitution process, during which the adsorbed water molecules on the metal surface H2O(ads) are replaced by inhibitor molecules Org(sol) [25].
where Org(sol) and Org(ads) are the organic molecules in the aqueous solutions and adsorbed on the metallic surface, respectively, H2O(ads) is the water molecules on the metallic surface, x is the size ratio representing the number of water molecules replaced by one molecule of organic adsorbate.
Attempts were made to fit these θ values to various isotherm including Frumkin, Langmuir, Temkin. The best fit was obtained with the Langmuir isotherm given by the equation:
where C is the inhibitor concentration, b is the equilibrium constant for the adsorption/desorption process. The relationship between C/θ and C at 303 K for mild steel in 1 M HCl and 0.5 M H2SO4 solutions with various concentrations of the plant extract is shown in Fig. 7 with slopes of 1.09 and 1.02, respectively. As can be seen by the good fit, RL extract as inhibitor obeys the Langmuir adsorption isotherm. The strong correlation coefficient (R 2 = 0.998) for the Langmuir adsorption isotherm plot confirms the validity of this approach.
Theoretical considerations
As it is often the case with biomass extracts, the multi-component nature of RLE makes it rather difficult to ascribe the observed inhibiting effect to any particular constituents. Nevertheless, since corrosion inhibiting action is often linked with the presence of polar heteroatoms, π-bond conjugation, aromaticity, electron delocalization regions, etc., it is possible to qualitatively predict the adsorption behavior of extract components having chemical structures similar to those of conventional organic molecules with previously established efficacy. Based on this consideration, the chemical structures of MMF, DMT and RBF recommend them for theoretical assessment of corrosion inhibiting potential. The quantum-chemical computations are not necessarily intended to provide detailed description of the adsorption of the extract. Instead, the idea is to recognize the relative contributions of the different extract components through their individual adsorption strengths and mechanisms.
The optimized structures, the HOMO and LUMO orbital distributions for the three major RL active components are shown in Fig. 8. Table 6 provides some quantum-chemical parameters related to the molecular electronic structure of the most stable conformation of the molecules. According to the frontier orbital theory, E HOMO is often associated with the electron donating ability of a molecule. High values of E HOMO indicate a tendency of the molecule to donate electrons to appropriate acceptor molecules with low-energy, empty electron orbital. Similarly, E LUMO represents the ability of the molecule to accept electrons. The lower the values of E LUMO, the more probable it is that the molecule would accept electrons [1, 29, 30]. ΔE, the difference of E LUMO and E HOMO, is another important factor that should be considered. It has been reported that low values of ΔE will provide good IE, because the energy for removing an electron from the last occupied orbital will be low [51].
Optimized structures and molecular orbital plots for: a MMF, b DMT and c RBF (atom legend: white H, gray C, red O. The blue and yellow isosurfaces depict the electron density difference, the blue regions show electron accumulation, while the yellow regions show electron loss. The green isosurfaces describe the Fukui functions, color online)
In Table 7, the values of E HOMO of the compounds under consideration increased in the following order: RBF > DMT > MMF. The values of ΔE decreased in the following order: RBF > MMF > DMT. The observed trend is not unexpected when we consider the structure and size of the three compounds. RBF has the size advantage as well as the presence polar heteroatoms (N, O), π-bond conjugation, and aromaticity, the features that enhance adsorption on metal surface and hence IE. Between MMF and DMT, though DMT is lager in size, the presence of π-bond probably made the difference.
Molecular dynamics (MD) simulations were undertaken to model the adsorption of the molecules on the metal surface at a molecular level. This was achieved using Forcite quench MD in the MS Modeling 4.0 software to sample many different low energy configurations and identify the low energy minima [36, 37]. Optimized structures of RBF, DMT and MMF and the Fe surface were used for the simulation. Calculations were carried out in a 9 × 7 supercell using the COMPASS force field and the Smart algorithm. The Fe crystal was cleaved along the (110) plane. Temperature was fixed at 350 K, with NVE (microcanonical) ensemble. The time step was 1 fs and simulation time 5 ps. The system was quenched every 250 steps. The optimized (lowest energy) adsorption model for single molecule of RBF on the Fe (110) surface from the simulation is shown in Fig. 9. The molecules can be seen to maintain a flat-lying adsorption orientation on the Fe surface, with the regions of high HOMO density acting as the adsorption sites. Using the quench MD method above, we calculated the binding energy (E Bind) of each molecule on the Fe surface using the following equation:
E Mol, E Fe and E total correspond respectively to the total energies of the molecule, Fe (110) slab and the adsorbed Mol/Fe (110) couple. In each case the potential energies were calculated by averaging the energies of the five structures of lowest energy, and a negative value of E Bind corresponds to a stable adsorption structure [52]. The obtained values of the binding energy were −126.40, −82.23 and −74.67 kcal/mol for RBF, DMT and MMF, respectively. The higher stability of the adsorbed RBF compared with MMF and DMT is attributable to the larger size as well as the structure of the RBF molecule, which makes a greater contribution to the observed inhibiting effect of RL extract.
Mechanism of inhibition
The first stage in the action mechanism of inhibitor in acid media is adsorption on the metal surface [53]. In most inhibition studies, the formation of donor–acceptor surface complexes between π-electrons of inhibitor and the vacant d-orbital of metal were postulated [25, 40].
The major constituent of extract of RL is RBF [28], whose structure is given in Fig. 8c. In the acid solution, due to protonation of amine group, the inhibitor molecules get adsorbed on the mild steel surface. The presence of heteroatoms atoms such N, O directly attached to the aromatic ring enhances the availability of π-electrons to get bonded to the vacant d-orbital of Fe. The high performance of RBF could also be due to large size of this molecule which covers wide areas on the metal surface and thus retarding the corrosion [53, 54]. However, a synergistic effect of this molecule with the other constituents may play important role on the overall IE of RL extract.
Materials preparation
Corrosion experiments were performed on mild steel specimens with weight percentage composition as follows; C, 0.05; Mn, 0.6; P, 0.36; Si, 0.3 and the balance Fe. The aggressive solutions were respectively 1 M HCl and 0.5 M H2SO4 solutions prepared from analytical grade reagents. Stock solutions of RL extract were prepared by boiling weighed amounts of the dried and ground leaves of RL in 1 M HCl and 0.5 M H2SO4 solutions, respectively under reflux for 3 h. The resulting solutions were cooled then triple filtered. The amount of plant material extracted into solution was quantified by comparing the weight of the dried residue with the initial weight of the dried plant material before extraction. From the respective stock solutions, inhibitor test solutions were prepared in the desired concentration range by diluting with the corresponding aggressive solution.
Conclusion
RL functioned as a good inhibitor for the corrosion of mild steel in 1 M HCl and 0.5 M H2SO4 solutions and the inhibiting efficiency value increased with the inhibitor concentration. The electrochemical impedance study showed that corrosion inhibition of mild steel in both HCl and H2SO4 solutions takes place by adsorption process. Tafel polarization curves indicated that the RL acted as mixed type in HCl but predominantly cathodic in H2SO4 solution. The adsorption of the RL on the mild steel surface in acid solution obeyed Langmuir adsorption isotherm.
References
Bahrami MJ, Hosseini SMA, Pilvar P (2010) Experimental and theoretical investigation of organic compounds as inhibitors for mild steel corrosion in sulfuric acid medium. Corros Sci 52:2793–2803
Zhang QB, Hua YX (2009) Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim Acta 54:1881–1887
Abdalla M (2004) Guar gum as corrosion inhibitor for carbon steel in sulphuric acid solutions. Port Electrochim Acta 22:161–175
Zhang DQ, Cai QR, Gao LX, Lee KY (2008) Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid. Corros Sci 50:3615
Ozcan M, Solmaz R, Kardas G, Dehri I (2008) Adsorption properties of barbiturates as green corrosion inhibitors of mild steel in phosphoric acid. Colloid Surf A 325:57–63
Talati JD, Gandhi DK (2004) Triphenylmethane dyes as corrosion inhibitors for aluminium–copper alloy in HCl acid. Mater Corros 33(3):155–163
Prabhu RA, Venkatesha TV, Shanbhag AV (2009) Carmine and fast green as corrosion inhibitors for mild steel in hydrochloric acid solution. J Iran Chem Soc 6(2):353–363
Beaza H, Guzman M, Ortega P, Vera L (2003) Corrosion inhibition of copper in 0.5 M hydrochloric acid by 1,3,4-thiadiazole-2,5-dithiol. J Chil Chem Soc 48(3):23–30
Ebenso EE, Oguzie EE (2005) Corrosion inhibition of mild steel in acidic media by some organic dyes. Mater Lett 59:2163–2165
Ali SA, Saeed MT, Rahman SU (2003) The isoxazolidines: a new class of corrosion inhibitors of mild steel in acidic medium. Corros Sci 45:253–266
Yıldırım A, Çetin M (2008) Synthesis and evaluation of new long alkyl side chain acetamide, isoxazolidine and isoxazoline derivatives as corrosion inhibitors. Corros Sci 50:155–165
Bartos M, Hackerman N (1992) A study of inhibition action of propargyl alcohol during anodic dissolution of iron in hydrochloric acid. J Electrochem Soc 139:3428–3433
De Souza FS, Spinelli A (2009) Caffeic acid as a green corrosion inhibitor for mild. Corros Sci 51:642–649
Da Trindade LG, Gonçalves RS (2009) Evidence of caffeine adsorption on low-carbon steel surface in ethanol. Corros Sci 51:1543–1578
Raja BP, Sethuraman MG (2008) Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater Lett 62:2977–2979
El-Etre AY (2006) Khillar extact as inhibitor for cid corrosion of SX 316 steel. Appl Surf Sci 252:8521–8525
Oguzie EE (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild Steel. Corros Sci 50:2993–2998
Okafor PC, Ikpi ME, Uwaha IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllantus amaratus extracts on the corrosion of mild steel in acidic media. Corros Sci 50:2310–2317
Gunasekaran G, Chauhan LR (2004) Eco friendly inhibitor for corrosion inhibition of mild steel in phosphoric acid medium. Electrochim Acta 49:4387
Chauhan LR, Gunasekaran G (2007) Corrosion inhibition of mild steel by plant extract in dilute HCl medium. Corros Sci 49:1143–1161
Da Rocha JC, Da Cunha Ponciano Gomes JA, D’Elia E (2010) Corrosion inhibition of carbon steel in hydrochloric solution by fruit peel aqueous extracts. Corros Sci 52:2341–2348
Lebrini M, Bentiss F, Vezin H, Lagrenee M (2006) The inhibition of mild steel corrosion in acidic solutions by 2,5-bis(4-pyridyl)-1,3,4-thiadiazole: structure–activity correlation. Corros Sci 48:1279–1291
El-Etre AY (2007) Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves. J Colloid Interface Sci 314:578–583
Satapathy AK, Gunasekaran G, Sahoo SC, Amit K, Rodrigues PV (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856
Quraishi MA, Singh A, Singh VK, Yadav DK, Singh AK (2010) Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater Chem Phys 122(2010):114–122
Rajendran S, Jeyasundari J, Usha P, Selvi A, Narayanasam B, Regis AP, Regan P (2009) Corrosion behaviour of aluminium in the presence of an aqueous extract of Hibiscus rosasinensis. Port Electrochim Acta 27(2):153–164
Jansen PCM, Cardon D (2005) PROTA, 3: dyes and tannins/colorants, [CD-Rom]. PROTA, Wageningen
Bringmann G, Ochse M, Wolf K, Kraus J, Peters K, Peters EM, Herderich M, Ake Assi L, Tayman FK (1999) 4-Oxonicotinamide-1-(1′-b-d-ribofuranoside) from Rothmannia longiflora Salisb. (Rubiaceae). Phytochemistry 51:271–276
Ju H, Kai ZP, Li Y (2008) Aminic nitrogen-bearing polydentate Schiff base compounds as corrosion inhibitors for iron in acidic media: a quantum chemical calculation. Corros Sci 50:865–871
Sahin M, Gece G, Karcı F, Bilgic S (2008) Experimental and theoretical study of the effect of some heterocyclic compounds on the corrosion of low carbon steel in 3.5% NaCl medium. J Appl Electrochem 38:809–815
Akalezi CO, Enenebaku CK, Oguzie EE (2012) Application of aqueous extracts of coffee senna for control of mild steel corrosion in acidic environments. Int J Ind Chem 3:13
Macdonald RJ (1992) Impedance spectroscopy. Ann Biomed Eng 20:289–305
Cruz J, Pandiyan T, Ochoa EG (2005) A new inhibitor for mild carbon steel: electrochemical and DFT studies. J Electroanal Chem 583:8
Lopez DA, Simison SN, de Sanchez SR (2005) Inhibitors performance in CO2 corrosion: EIS studies on the interaction between their molecular structure and steel microstructure. Corros Sci 47:735
Seifzadeh D, Rajabalizadeh Z (2013) Environmentally-friendly method for electroless Ni–P plating on magnesium alloy. Surf Coat Technol 128:119–126
Sorkhabi HA, Shaabani B, Seifzadeh D (2005) Effect of some pyrimidinic Schiff bases on the corrosion of mild steel in hydrochloric acid solution. Electrochim Acta 50:3446
Lebrini M, Robert F, Lecante A, Roos C (2010) Corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros Sci 53(2):687–695. doi:10.1016/j.corsci.2010.10.006
Khaled KF, Abdel-Rehi SS (2011) Electrochemical investigation of corrosion and corrosion inhibition of iron in hydrochloric acid. Arab J Chem 4:397–402
Sam JK, Mohammad A, Abraham J (2011) Electrochemical, surface analytical and quantum chemical studies on Schiff bases of 4-amino-4H-1,2,4-triazole-3,5-dimethanol (ATD) in corrosion protection of aluminium in 1 N HNO3. Bull Mater Sci 34(6):1245–1256
Muralidharan S, Quraishi MA, Iyer SKV (1995) Effect of molecular structure on hydrogen permeation and the corrosion inhibition of mild steel in acidic solutions. Corros Sci 37(11):1739–1750
Popova A, Sokolova E, Raicheva S, Christov M (2003) AC and DC study of the temperature effect on the mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros Sci 45:33–58
Szauer TA, Brandt A (1981) Adsorption of oleates of various amines on iron in acidic solution. Electrochim Acta 26:1253–1256
Martinez S, Stern I (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Putilova IN, Balezin SA, Baranik UP (1960) Metallic corrosion inhibitor. Pergamon Press, New York, p 31
Bochris JMO, Reddy AKN (1997) Modern electrochemistry, vol 2. Plenum Press, New York, p 1267
Bhajiwala HM, Vashi RT (2001) Ethanolamine, diethanolamine and triethanolamine as corrosion inhibitors for zinc in binary acid mixture (HNO3 + H3PO4). Bull Electrochem 17:441–448
Gomma GK (1998) Corrosion of low carbon steel in sulphuric acid in presence of furan-halide mixtures. Asian J Chem 5(3):761
Makhlouf MTh, Gomma GK, Wahdan MH, Khali ZH (1995) Effect of cyanine dye–solvent interaction on the electrochemical corrosion behavior of low carbon steel in acid medium. Mater Chem Phys 40:119
Gomma GK, Wahdan MH (1994) Effect of temperature on the acidic dissolution of copper in the presence of amino acids. Mater Chem Phys 39(2):142–148
Quraishi MA, Sardar R (2003) Hector bases—a new class of heterocyclic corrosion inhibitors in acid solutions. J Appl Electrochem 33:1163–1168
Khaled KF (2010) Corrosion control of copper in nitric acid solutions using some amino acids—a combined experimental and theoretical study. Corros Sci 52:3225–3234
Ogukwe CE, Akalezi CO, Chidiebere MA, Oguzie KL, Iheabunike ZO, Oguzie EE (2012) Corrosion inhibition and adsorption of Anthocleista djalonesis leaf extract on the acid corrosion of mild steel. Port Electrochim Acta 30(3):189–202
Quraishi MA, Singh A, Singh VK, Yadav DK, Singh AK (2010) Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater Chem Phys 122:114–122
Trabenelli G, Mansfeld F (eds) (1987) Corrosion mechanisms. Marcel Dekker, New York, p 109
Acknowledgments
This project is supported by TWAS, The Academy of Science for the developing world, under the TWAS Grants for Research units (TWAS-RGA08-00).
Authors’ contributions
COA and CEO carried out the weight loss and polarization experiments, and drafted the manuscript. EEO carried out the theoretical study and sequence alignment. AEA proof read and edited the manuscript. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Akalezi, C.O., Oguzie, E.E., Ogukwe, C.E. et al. Rothmannia longiflora extract as corrosion inhibitor for mild steel in acidic media. Int J Ind Chem 6, 273–284 (2015). https://doi.org/10.1007/s40090-015-0050-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0050-z