Abstract
Various concentrations of copper are embedded into silica matrix to xerogels form using copper source—Cu(NO3)2·3H2O. The xerogel samples are prepared by hydrolysis and condensation of tetraethyl orthosilicate (TEOS) with determination of new molar ratio of components by the sol–gel method. CuO/SiO2 nanocomposite is prepared with mixture of components with new molar ratio of H2O/TEOS to be 6.2. In this investigation, the necessary amount of trihydrated copper nitrate is added to the solution in such a manner that the concentration of the copper oxide in final solution reaches 1, 2 and 5 wt% (samples A, B and C, respectively). After ambient drying, the gel samples are heated from 60 to 1,000 °C at a slow heating rate (50 °C/h). Thermal treatment effect is characterized by Fourier transmission infrared, thermal gravimetric analysis, X-ray diffraction, transmission electron microscopy, scanning electron microscopy, surface analysis and thermal program reduction methods at different temperatures.
Similar content being viewed by others
Introduction
Organic and inorganic materials molecular chemistry has made a great progress during the last few decades. Apart from polymer materials, most ceramic and glass materials are prepared by solid-state reactions. The main exception, when the synthetic methods are based on a molecular chemistry approach, is the preparation of oxides by sol–gel method [1]. The sol–gel method has been well studied and developed in material science since they are well suited for preparing and designing devices with very specific properties [2]. The sol–gel processes may be divided into two classes depending on the nature of the precursors: inorganic precursors (chlorides, nitrate, sulfides, etc.) and alkoxide precursors. The route that involves the use of alkoxide precursors appears as the more versatile one [3]. Figure 1 exhibits total pattern of sol–gel process. Sol–gel method is a relatively new technique for preparation of glasses to get high purity and homogeneity at low temperatures processing [4]. Introducing transition metal into hydrolysable precursors through sol–gel methods permits the formation of numerous novel materials that exhibit important optical and/or catalytic properties [5, 6]. Copper or copper oxides in oxide matrixes have attracted sustained interest due to their unusual properties [7]. Mohanan and Brock [8] have studied copper oxide silica aerogel composites by varying pH values, copper precursor salts, and treatment temperatures. They found that base-catalyzed gels underwent a gradual change from bonded Cu+2 to segregated CuO at different heating conditions. Parler et al. [9] observed silicon–oxygen–metal bond formation during both synthesis and drying stages at low temperatures with relative high copper concentration. Some properties such as bacterial inhibitory capacity depend highly on the copper ion complex state at lower concentrations [10]. In this study, host matrices have been extensively embedded with copper that was produced with different copper concentrations (1, 2 and 5 wt%) and examined during gelation and drying. The characterization of the xerogels are examined by UV–Vis, Fourier transmission infrared spectroscopy (FTIR), thermal gravimetric analysis (TGA), transmission electron microscope (TEM), scanning electron microscope (SEM), X-ray diffraction (XRD), BET and temperature-programmed reduction (TPR) methods at different temperatures [11].
Experimental procedure
Materials
In this investigation the raw materials consist of tetraethyl orthosilicate (TEOS) (Fluka, 98 %), ethanol absolute (EtOH) (Merck), copper nitrate trihydrated (Merck), HNO3 (Merck, 65 %), CH3COOH (Merck, 99–100 %) were used with mentioned specification.
Sample preparation
Three samples of CuO/SiO2 nanocomposite in xerogel form were prepared by the sol–gel method and another one was prepared as a blank sample using TEOS hydrolyzed with HNO3 and CH3COOH, ethanol and deionized water (DI) with new total molar ratio of TEOS:ETOH:H2O = 1:1.3:6.2 [8]. The necessary amount of trihydrated copper nitrate was added to solution that the concentrations of copper oxide in the final product reach 1, 2 and 5 wt% (samples A, B and C, respectively). Then the solutions were mixed by magnetic stirrer for 1.5 h, to make them more homogeneous. The solution acidity (pH) was measured by pH-meter which was reported as 2.4. The samples are kept in a close container at 25–30 °C temperature. The soft gel was prepared till dark-blue color appears along 84 h (3.5 days) via gelation treatment. Then the gel samples are dried in an oven at 60–100 °C temperature in air atmosphere for 1 h, to complete the gelation process. The gel samples are annealed by electrical furnace in the range of 100–1,000 °C at 50 °C/h for 2 h, to form the condense nanocomposites [12]. The blank solution is prepared without addition of Cu(NO3)2·3H2O with the mention methods.
Characterization
UV–Vis spectrum is determined by UV spectrophotometer type of BEKMAN (DU-600).
Fourier transmission infrared spectroscopy spectra, was carried out by a Genesis system, model ATI, using 0.05 g of powder sample with 0.3 g of KBr.
Temperature-programmed reduction was used to investigate the availability of copper species for the reaction with gas-phase molecules. These results can be correlated with catalytic activity such as carbon monoxide oxidation. The sol–gel samples were used for the TPR directly after the thermal treatment with no pre-activation. For TPR experiments, 20 mg samples in a quartz tube under H2 (8 %)/N2 flow were heated at 10 °C min−1 up to 700 °C and the H2 consumption monitored by a thermal conductivity detector (TCD).
Thermal gravimetric analysis was obtained by Rheometric Scientific instrument, model STA 1500.
The acidity of solution (pH) was measured by Omega pH-meter, model 744.
The condensation and annealing of the samples was done in an Oxaiton heat furnace with high thermal capacity (1,500 °C).
Structural analysis
A TEM Em208S series, made by Phillips Company, operating at 100 kV was used for this investigation. The dry samples were ground suspended in dry cyclohexane and sonicated for 30 min. Then the solutions were allowed to settle and a droplet of the resulting supernatant was placed on a holey carbon film and dried.
A SEM XL-30 series, made by Phillips Company, was used. The samples were powdered and coated with gold, before the examination by SEM. The XRD patterns were performed in a Philips diffractometer model PW1800 using copper anodic tube with wavelength of 1.54 Å.
The porosities of the samples ere analyzed by nitrogen adsorption/desorption measurements fitted to a BET isotherm using an Autosorb instrument (Quanta-chrome, Nova 1200). The samples were pre-treated for 3 h under vacuum at 100 °C (for the sample dried at 100 °C) and at 200 °C (for samples treated at 400, 800 and 1,000 °C). Gaseous nitrogen was used with a 5-h adsorption/desorption cycle.
Results and discussion
The bonding and molecular structure property of CuO/SiO2 nanocomposite was done in bonding vibrational mode by FTIR spectrum. Figure 2 presents the FTIR absorbance spectrum in the range of 500–4,000 cm−1 for SiO2:CuO in the powdered sample. Figure 2 is the FTIR spectrum of copper ions (5 wt%) doped in silica matrix heated at 500 and 900 °C. In this figure, it can be seen that the hydroxyl groups are removed at 900 °C, although molecular water can be taken away at about 200 °C. However, hydroxyl groups can be removed in temperature range of 800–1,000 °C.
The absorption band in range of 1,450 cm−1 related to reduction of nitrate groups in the solution with increasing temperature, presents complete decomposition of these groups at 900 °C. The main bond of the SiO2 FTIR spectrum is due to the asymmetric stretching mode at 1,150–1,250 cm−1; whereas, this sharp band shifts to lower wave number at 900 °C [13]. This is clear that the absorption band in 900–1,000 cm−1 is assigned to Si–OH groups, due to these groups often disappearing in heated pure silica. FTIR spectrum of CuO/SiO2 xerogel sample presents that the absorption bands in 950 and 1,200 cm−1 are separated from each other.
The TGA analysis of Cu(NO3)2/SiO2 at 30–300 °C temperatures is presented in Fig. 3, as well. There is a weight loss about 1 mg at 30–100 °C, due to the evaporation of residual alcohol and water at 100 °C. In the temperature range of 100–200 °C, the weight loss is constant and no significant weight loss was observed at these temperatures, but at the range of 200–300 °C, we see a small weight decrease, probably related to the thermal decomposition of surface hydroxyl and ethoxyl groups [14].
Structural analysis
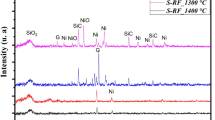
The microstructure of the xerogel was examined by TEM. Powder samples with 5 wt% copper after ambient drying and thermal treatment at 400 °C in air for 1 h, were subjected to TEM using bright field, and the resulted image is shown in Fig. 4. No crystalline species were detected without thermal treatment, and bright field image shows a typical amorphous xerogel. After heating at 400 °C, copper species start to segregate. This figure confirms the formation of average size of about 50 nm. Figure 5 presents the X-ray pattern of the set of the CuO/SiO2 samples annealed at 200, 400 and 600 °C. For the 200 °C sample, the pattern corresponding to the copper nitrate is still presented as the partially hydrolyzed phase Cu2(OH)3(NO3).
Although the amount of the added copper nitrate is below the limit of solubility and all precautions were taken to dissolve it, two states could occur during the thermal treatment of the gel, which explain the later fact that the precipitation of the compounds or separated ions lead to formation of the compounds during the annealing. For the samples annealed at 400 and 600 °C (A), it can be seen that the X-ray pattern corresponds to the CuO phase in Tenorite structure [15]. From these results it can be said that there is no evidence of the presence of Cu2O species in these samples, but for the samples B and C, there is no crystalline structure at these temperatures.
The backscattered electron images of the samples present well-defined regions with 5 wt% copper by SEM at 100 °C that exhibit in Fig. 6. At 100 °C, it can be seen that the copper nitrate particles are embedded in the silica matrix, but with increasing temperature at 400–800 °C, the copper nitrate particles are decomposed and converted to copper oxide particles that doped into the silica matrix. By thermal treatment at 1,000 °C, the copper oxide particles are decomposed and form an oxide network containing metal oxide clusters like Cu–O–Cu that are connected to silica support. This process completely depends on the thermal treatment effects and copper source. These metallic nano-clusters have a structure with excellent stability and reproducibility [14]. It is reported that interaction of support and metal is performed by hydrogen atom, i.e., metal or metal oxide interacts with hydroxyl groups. We can say that copper ions are doped into silica matrix to form copper nitrate and there are no more copper oxide particles. But with raising temperature and decomposition of copper nitrate, the copper oxide particles are formed and interact with silica matrix by OH. It can also be concluded that there is a relation between existence of copper oxide in silica matrix, and overlapping of silanol groups and Si–O bands in infrared spectrum. This overlapping leads to interaction between guest particles and host support.
These results indicate the loss of volatile compounds from the porous structure. Once the volatile compounds are eliminated from the network, an increase occurs in the porosity and the gas pore interface. Despite this weight loss, a little shrinkage is observed for the material treated at 400 °C, resulting in density decrease. It is interesting for the sample treated at 400 °C, the surface area increases by 38 % (from 254 to 351 m2 g−1); whereas, the pore volume shows a much more pronounced increase of 211.7 % (from 196 to 611 cm3 g−1). For the sample treated at 800 °C, the surface area decreases by 53.5 %, whereas the pore volume shows reduction of 76.6 %. Also, for the sample treated at 1,000 °C, a surface area decrement of 95 % is observed; whereas, the pore volume shows a reduction of 92.3 % and a porosity decrease of 64.3 % (from 14 to 5 Å). Although, this effect is not clear, in authors’ view it is related to the size and shape of micro-, meso- and macro-porous, which modify as the sample is treated at different temperatures. For the higher temperatures (800 and 1,000 °C) the surface area and porosity strongly decrease due to densification process. The results have presented a significant contribution of micro-porous in the material treated at 100 and 400 °C. For higher thermal treatment, the surface area decreases due to the presence of micro- and meso-porous, but the contribution of the meso-porous area in the total surface progressively increases [16] (Table 1).
TPR studies
Thermal program reduction experiments show the reduction of copper species to Cu0 by H2. Both Cu+2 and Cu+1 are reduced directly in a single step to form Cu0 according to the reactions Cu+2 → Cu0 and Cu+1 → Cu0. TPR analysis of the samples treated at different temperatures is presented in the Fig. 7. The TPR profile of the sample Cu100 shows three hydrogen consumption peaks; a large peak at (1) 325 °C, two minor ones at (2) 280 and (3) 245 °C. For sample Cu400, the TPR profile is shifted at ~25 °C to lower temperatures. The largest peak observed for sample Cu400 at (1) 290 °C was much smaller and the other peaks at (2) 255 and (3) 220 °C increased in intensity. These results indicate that the peak (1) is related to the reduction of Cu(NO3)2 and the peaks (2) and (3) are due to the reduction of copper oxides. When the sample was treated at 800 °C, TPR profiles were shifted back to higher temperature and peak (1) related to Cu(NO3)2 was absent, whereas peaks (2) and (3) related to copper oxides, increased strongly in intensity. At 1,000 °C, TPR profiles present that the peaks (2) and (3) are mixed together and form a broad peak. It can also be observed that the hydrogen consumption decreases as treatment temperature increases, according to the results indicated in Table 2.
Samples at annealed at 800 and 1,000 °C only have 46 and 15 % of amount of copper species available for reduction, in comparison to the samples at annealed at 100 and 400 °C, respectively. These results could be related to the reduction of copper by the organic compounds present in the silica matrix during the heating treatment. Moreover, all thermal treatments were carried out in air and a reduced copper form such as Cu0 would be unlikely. To explain the decrease in TPR of H2 consumption, one can envisage that treatment at temperatures higher than 800 °C, the densification process with sintering and closing of the pores may lead to an entrapment of copper species in the vitreous silica matrix. The entrapped copper species will not be available on the surface for the reaction with H2 during TPR experiments.
Conclusion
Fourier transmission infrared spectroscopy measurements confirm the removal of hydroxyl groups from the samples at 900 °C temperature. TEM micrograph confirms the formation of colloidal particles of about 50 nm size. Also, X-ray results shows that for CuO/SiO2 samples, there is formation of the copper clusters mainly in the form of a Tenorite. The evolution of the copper species goes from copper nitrate to copper oxide species by thermal treatment. It has been reported that the metal–support interaction is carried out by hydrogen that is the metal or metal oxide particles interact via OH groups. This fact can be corroborated by FTIR results. It is well known that the band at 850–990 cm−1 attributed to the Si–OH groups and The FTIR results show that the copper ions are mainly incorporated into the SiO2 matrix in copper nitrate form, and there are not copper oxide (CuO) particles, or their concentration is too low. After decomposition of the copper nitrate, the CuO particles are formed and they interact with the SiO2 matrix via OH groups. In the authors’ view, there is a correlation between the existence of copper oxide particles embedded into the silica matrix, and the overlapping of the silanol and Si–O stretching bond. This overlapping might be associated with the interaction between the guest particles and the matrix host [17]. According to this study, the exact composition of clusters depends on the annealing temperatures and the choice of appropriate copper source.
References
Sakka S, Kamiya K (1980) Glasses from metal alcholates. J Non-Cryst Solids 42:403
Livage J, Henry M, Sanchez C (1988) Sol–gel chemistry of transition metal oxides. Progr Solid State Chem 18:259
Hench L, Larry HL, West JK (1990) The sol–gel process. Chem Rev 90:33
Calderon-Guillen JA, Aviles-Arellano LM, Perez-Robles JF, González-Hernandez J (2005) Surf Coat Technol 190:110
Rolison DR, Dunn B (2001) Electrically conductive aerogels new materials in electrochemistry. J Mater Chem 11:963
Tseng I, Chang W, Wu CS (2002) Various sol–gel procedures and post-treatment were applied to modify the distribution of Cu. Appl Catal 203:37
Karakassides MA, Bourlions A, Petridis D (2000) Synthesis and characterization of copper containing mesoporous silica. J Mater Chem 10:403
Mohanan JL, Brock SL (2003) Sol–gel processing of semiconducting metal xerogels. Chem Mater 15:2567
Parler CM, Ritter JA, Amiridis MD (2001) Infrared spectroscopic study of sol–gel derived mixed metal oxides. J. Non Cryst Solids 279:119
Perez-Robles JF, Gonzales-Hernandez J (1999) Characterization of sol–gel glasses with different copper concentration treated under oxidizing and reducing condition. J Phys Chem Solids 60:1729
Martinez JR, Palomares-Sanchez S, Ortega-Zarzosa G, Ruiz F, Chumukov Y (2006) Rietveld refinement of amorphous SiO2 prepared via sol–gel method. Mater Lett 60:3526
De Sousa EMB, Porto AO, Schilling PJ, Alves MCM, Mohallem NDS (2000) Study of the structural evolution of copper-doped porous silica-gels. J Phys Chem Solids 61:853
Martinez JR, Ruiz F, Vorobiev YV, Gonzalez-Hernandez J (1998) Rietveld refinement of amorphous SiO2 prepared via sol–gel method. J Chem Phys 109:7511
Wang Z, Liu Q, Yu J, Wu T, Wang G (2003) Surface structure and catalytic behavior of silica supported copper catalyst prepared by impregnation. Appl Catal A 239:87
International Center for diffraction data, inorganic phases (1993)
Martinez JR, Ruiz F, Vorobiev YV, Gonzalez-Hernandez J (1998) Maepo structural de silica xerogel utilizando espectroscopia. J Chem Phys 109:754
Ruiz F, Martinez JR, Gonzalez-Hernandez J (2000) Formation of silicate structures in Cu-containing silica. J Mater Res 15(12):2875
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Tohidi, S.H., Gholamzadeh, S., Shirazi, M.A.Z. et al. Characterization of sol–gel derived CuO/SiO2 nanostructure on temperature. Int J Ind Chem 5, 63–68 (2014). https://doi.org/10.1007/s40090-014-0017-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-014-0017-5