Abstract
The use of sweet potato, Ipomoea batatas flour as a carbon source for biofloc production in freshwater culture of tilapia, Oreochromis sp., was evaluated in terms of its effects on water quality, microbial population and fish yield. The experiment was conducted using 20 L glass aquaria with two sources of carbon, namely, wheat flour and sweet potato flour. Glass aquaria with no carbon source were used as control. The treatments and control were done in duplicate. Biofloc was produced in the glass aquaria for 10 days, followed by stocking of tilapia juveniles (ABW: 15 g) at a density of 4 fish/aquarium and reared for 30 days. During the rearing period, biofloc production was maintained by adding the different flours at a carbon:nitrogen (C:N) ratio of 16. Commercial feeds (35 % crude protein) were also given at 6 % body weight. Moderate aeration was provided in all aquaria to maintain the flocs in suspension. The levels of total suspended solids and biochemical oxygen demand as well as total heterotrophic bacteria and bacilli populations were significantly higher in the treatment groups than the control. However, the levels of nitrogenous wastes (ammonia-N, nitrite-N and nitrate-N) were generally lower in glass aquaria added with sweet potato flour. At the end of the experiment, there were no significant differences in the average weights of tilapias from the biofloc groups and the control, although the tilapias that were reared in the biofloc group with sweet potato flour had the lowest average body weight and growth rate. Tilapias from both biofloc groups had significantly lower feed conversion ratio compared with the control, with the wheat flour biofloc group performing better than the sweet potato flour biofloc group. Taken together, the results of this study showed that the bioflocs that are produced using sweet potato flour as a carbon source could lower nitrogenous waste in the rearing water and to a certain degree serve as food for tilapias in freshwater culture. Efficient feeding management is needed to ensure good growth of the fish if sweet potato flour is to be utilized for biofloc production.
Similar content being viewed by others
Introduction
High-yield, high-density aquaculture is a rapidly growing industry and is accompanied with increased pollution of the aquatic environments (De Schryver and Verstraete 2009). Aquaculture animals usually absorb 20–25 % of protein in the feed, with the rest discharged into the water in the form of ammonia nitrogen, food remains and excrement, resulting in reduced water quality (Crab et al. 2007; Piedrahita 2003). High concentrations of ammonia–nitrogen and nitrite in aquaculture water also cause disease or even death of the farmed animals, which is a serious impediment to the development of the aquaculture industry (Yang and Qiu 2006).
Several new methods have been proposed to solve the problems associated with high concentrations of ammonia–nitrogen and nitrite in the water. Biofloc technology (BFT) is one of these methods, which have shown numerous advantages (Wang et al. 2015). The use of BFT in aquaculture has received considerable attention because the adoption of this technique results in high production yields, feed protein recycling, better water quality, and bacterial infection control (Avnimelech 2006; Crab et al. 2007; Little et al. 2008; Choo and Caipang 2015). The ideal candidates for BFT include shrimp, which are detritus feeders and tilapia, which are generally filter feeders (Azim and Little 2008). The basic principle of this technology is the generation of a nitrogen cycle in a stagnant aquaculture system by enhancing the growth of heterotrophic bacteria. This bacterial population utilizes nitrogenous wastes and subsequently serves as food for the cultured species (Avnimelech 1999; De Schryver et al. 2008). Biofloc systems have not only been shown to reduce nitrogenous wastes but they also improve the feed nutrient utilization efficiency and immunity of the cultured animals (Avnimelech 1999; Crab et al. 2009, 2012; Azim and Little 2008; Wang et al. 2015).
Maintaining an optimum carbon:nitrogen (C:N) ratio is crucial to the formation and production of bioflocs (Avnimelech 2006). High C:N ratio ranging 10–20 could promote the formation of biofloc as shown in previous studies (Avnimelech 1999; Hargreaves 2006; Asaduzzaman et al. 2008; Ballester et al. 2010). However, the C:N ratio in most commercial feeds is approximately 10; thus, additional carbon is required to increase the ratio and maintain biofloc production (Avnimelech 1999; De Schryver et al. 2008). The C:N ratio in feed could be increased by the addition of extra carbohydrate to the basic feed, or by the reduction of the crude protein content in feed (Wang et al. 2015). Although bioflocs have the capacity to augment the nutritional requirements of fish and shrimp in aquaculture, a number of studies have indicated that the ability of bioflocs to control the water quality in the culture system and their nutritional properties are largely influenced by the type of carbon source that was used to produce the flocs (Crab 2010; Crab et al. 2010). There are several organic carbon sources that could favor the growth of specific bacteria, protozoa and algae, and hence influence the microbial composition of the bioflocs (Crab 2010). These differences in the composition of the bioflocs also affect their nutritional properties (Crab 2010; Ekasari et al. 2010, 2014). Feeding experiments also demonstrated that the type of carbon source affected the availability, palatability and digestibility of these bioflocs (Crab 2010; Crab et al. 2010; Ekasari et al. 2010). For example, Ekasari et al. (2010) showed that regardless of the salinity, bioflocs that are produced using glycerol as a carbon source had higher total n-6 PUFAs than that of using glucose during the culture of tilapia. This was also shown by the studies done by Crab (2010). In shrimp culture, molasses is the most widely used carbon source for biofloc production (Baloi et al. 2013; Schveitzer et al. 2013; Correia et al. 2014). Previously, we compared wheat flour and corn flour as carbon sources for biofloc production in freshwater tilapia and showed that there were no significant effects on water quality between the biofloc groups and the control (Caipang et al. 2015). However, in terms of weight gain and feed conversion ratio (FCR), wheat flour performed better than corn flour, indicating that the former can be a good candidate as a source of carbon during biofloc production in freshwater tilapia culture. In spite of the popularity of molasses and wheat flour as carbon sources for biofloc production, further research should focus on the use of low-cost non-conventional agro-industrial products as carbon source. Different carbon sources stimulate the growth of the indigenous microbiota in various ways and would greatly influence the water quality, in situ feed production and utilization of the flocs by the cultured organisms. Various products or residues of the local industry can provide a low-cost external carbon source for application in biofloc technology in the culture of fish, but will need extensive research before implementation on a larger scale. Hence, this study evaluated the use of sweet potato (Ipomoea batatas) flour, an abundant and cheap source of carbon for biofloc production in terms of its effects on water quality, microbial population and yield during culture of tilapia in freshwater. The effects of sweet potato flour were compared against the performance of wheat flour to determine whether the former can be used as an alternative source of carbon for biofloc production in tilapia culture.
Materials and methods
Glass aquaria and experimental design
The experiment was carried out at the Aquaculture Workstation of Temasek Polytechnic, Singapore. Glass aquaria (20 L capacity) were cleaned, disinfected and sundried prior to the experiment. Each aquarium was filled with 1 L of water taken from Bedok Reservoir, Singapore, scaled up to 15 L using de-chlorinated freshwater and provided with aerators. After the addition of water, 100 mL of Chlorella (density of 106–108 cells mL−1) was added to each aquarium and supplemented with 8.5 mL of the algal nutrient. The treatments consisted of A: biofloc production using wheat flour as carbon source; B: biofloc production using sweet potato flour as carbon source and C: no biofloc production with no addition of flour. Each treatment was done in duplicate.
Biofloc production and fish stocking
For biofloc production in glass aquaria, the corresponding flour and commercial feed (crude protein of 35 %) combinations were added following a previously described procedure (Caipang et al. 2015). The amount of flour that was added together with the commercial feeds had a C:N ratio of 16:1 following the calculations of Crab et al. (2012). The use of the commercial feed for biofloc production in glass aquaria was to trigger the production of nitrogenous wastes and to be subsequently utilized by the heterotrophic bacteria that reside in the rearing water. Feeds and/or flour were added to each aquarium every morning at 9 AM. For the control group, 20 % of the total water volume was replaced every 2 days, while for the treatment groups, the water was replaced at the same volume as the control every 10 days or when the total suspended solids (TSS) exceeded 500 mg L−1. At the 11th day of the experiment, tilapia fingerlings with average weight of 15 g were stocked at a density of 4 fish in each aquarium (4 g L−1). The experiment lasted for 41 days.
Water quality monitoring
Water samples for determination of selected physico-chemical parameters were collected at the initial, 7th, 30th and 40th day of the experiment. Total ammonia–nitrogen (TAN), nitrite (NO2-N) and nitrate (NO3-N) from the water samples were determined using commercial water quality kits following the procedures of the manufacturer (Hach Company, Colorado, USA). TSS and biochemical oxygen demand (BOD) were analyzed using the procedures described in APHA (1989). The water samples for TSS and BOD determination were obtained and processed following previously described procedures (Caipang et al. 2015).
Bacterial counts
Total heterotrophic bacteria and bacilli from the water in all glass aquaria were enumerated on the 20th and 40th day of the experiment. The enumeration of total heterotrophic bacteria provided an estimate of the culturable bacterial population in the rearing water during biofloc production, while the bacilli counts would likely indicate the presence of potential probiotic candidates that could be utilized in further studies. The water samples were serially diluted using 1 × PBS and 100 µL of the various dilutions was plated onto both nutrient agar plates and MRS agar plates for the enumeration of total heterotrophic bacteria and bacilli, respectively. Each dilution was performed in duplicate. The plates were then incubated at 28 °C for 2 days and bacterial counts were expressed as log10 colony-forming units (CFU) mL−1.
Assessment of fish yield
At the end of the experiment, the weights of the tilapia were taken and recorded. The average body weight (ABW), average daily gain (ADG), specific growth rate (SGR) and feed conversion rate (FCR) were calculated from each group.
Data analyses
The data are represented as mean ± SD. One-way ANOVA was used to determine significant differences in the fish yield parameters among the different groups; thereafter, the differences between the groups were checked by Tukey’s Multiple Comparison Test. All significance levels were set at p < 0.05.
Results and discussion
Physico-chemical parameters of the rearing water
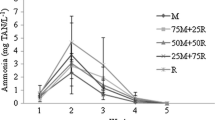
The total ammonia–nitrogen concentration in the wheat flour treatment group was higher compared with the control and the sweet potato treatment group (Fig. 1a). There was a large degree of fluctuation in the NO2-N concentration throughout the experiment for all the treatment groups with no observable trend among the treatment groups and the control (Fig. 1b). However, at the last sampling date, the NO2-N concentration in the sweet potato flour group was significantly lower compared to the wheat flour group and the control. The levels of NO3-N were generally lower in the sweet potato group than the wheat flour group and the control during the duration of the experiment (Fig. 1c).
These results suggest that sweet potato as a carbon source for biofloc production in a small-scale level might result in better water quality due to a reduction in the levels of the various forms of nitrogenous wastes within the system when this particular flour was used as a carbon source. In a previous study using wheat flour and corn flour as sources of carbon for small-scale biofloc production in freshwater tilapia showed variability in the levels of ammonia, nitrate and nitrite following addition of these carbon sources (Caipang et al. 2015). How these differences in the fluctuations of the nitrogenous wastes following the addition of these carbon sources related to the type of flour remain to be supported by further studies. Until such thorough experimentation is done, no firm conclusion can be advanced with regard to the best carbon source that could help mitigate the occurrence of high levels of nitrogenous wastes in freshwater BFT system for tilapia. However, the present study showed that the use of sweet potato in small-scale production of biofloc in freshwater tilapia culture seemed to be beneficial in lowering nitrogenous wastes in comparison with using either wheat or corn flour.
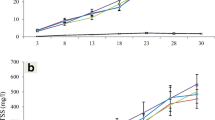
The total suspended solids (TSS) in both the biofloc treatment groups were higher compared to the control (Fig. 2a). The levels of the biochemical oxygen demand (BOD) as shown in Fig. 2b corresponded to the TSS concentration, where higher readings for both parameters were obtained in both treatment groups compared to the control. The results obtained were similar to the findings of Azim and Little (2008), where TSS concentrations and BOD levels are higher in the BFT tanks compared to the control. Caipang et al. (2015) also obtained similar trends for the levels of TSS and BOD when they used wheat and corn flour as carbon sources for small-scale biofloc production in freshwater tilapia culture. Regardless of the type of carbon source, addition of such will result in higher BOD and TSS in BFT systems.
Bacterial load
In Fig. 3a, it can be observed that total bacteria counts in the BFT groups were higher than the control throughout the duration of the experiment. This could be credited to the increased in bacteria population growth from the addition of carbon source in the BFT tanks (Avnimelech 1999). Similar results were also obtained in our previous study using wheat and corn flour as carbon sources for biofloc production in freshwater tilapia (Caipang et al. 2015). For the bacilli count, the wheat flour biofloc group has a consistently high bacilli population throughout the experiment period as shown in Fig. 3b. However, bacilli count was on a constant decrease in the sweet potato treatment, reaching a point where bacilli count was close to those of the control group at the last sampling date. An earlier study on the use of various carbon sources for biofloc production revealed that the organic carbon source of choice determines the composition of the flocs produced (De Schryver et al. 2008). This could have an effect on the bacterial populations that are closely associated with the flocs. The decreased population of bacilli in the group added with sweet potato flour could be an indication that this particular type of flour may not be a good source of carbon to maintain a stable population of bacilli compared to wheat flour. Additional research is needed to support the present study.
The presence of higher bacteria population in the BFT group as a result of the addition of carbon source could have resulted in the increased volume of the flocs. Higher biofloc production triggered the increase in TSS and a subsequent rise in respiration, which led to higher BOD levels. Previous studies on the use of bioflocs in tilapia culture are in agreement with the findings of the present study (Azim and Little 2008; Crab et al. 2010; Caipang et al. 2015). It is interesting to find out whether a correlation exists between bacterial population and the levels of nitrogenous wastes in the rearing water as this relationship could provide insight on the possible role of these bacteria in the dynamics of nitrogenous waste in the water. Such relationship was not established in the present study because there were differences in the sampling dates for determination of nitrogenous wastes and the enumeration of bacterial counts. However, this link between the two parameters will be the point of focus in future studies involving the use of BFT in tilapia culture.
Fish yield and growth parameters
Feed conversion rate (FCR) was significantly lower in the treatments compared to the control, with the FCR in the wheat flour treatment being lower compared to the sweet potato flour treatment (Table 1). The average daily gain (ADG; g day−1) in the wheat flour BFT treatment was almost similar to the control, while the ADG in the sweet potato flour was lower compared to both the control and wheat flour BFT treatment. The specific growth rate (SGR) of tilapia in the sweet potato treatment was lower than the control and wheat flour BFT treatment. No fish mortality was observed in both the BFT and non-BFT groups during the monitoring period.
The results obtained indicate that sweet potato flour as a carbon source could result in lower FCR compared to conventional tanks fed with commercial feed, suggesting the increased utilization of protein in these treatments. However, FCR in the sweet potato treatment is higher compared to the wheat flour treatment. Relatively slow growth rate was also observed in the sweet potato treatment compared to both control and wheat flour treatment. These could be credited to nutritional differences in flocs produced between the wheat flour treatment and sweet potato flour treatment, resulting in slower growth in fish feeding on flocs produced with sweet potato flour. This is supported by the study of Ekasari et al. (2010), which shows that there is a difference in nutritional content of biofloc produced by different carbon source. Further research on the nutritional difference in flocs between the two treatments is needed in this aspect.
Conclusion
In conclusion, small-scale studies showed that the bioflocs produced during the addition of sweet potato flour as a carbon source could lower nitrogenous waste and to a certain degree serve as food for tilapias in freshwater culture. However, to ensure that the fish are able to attain the desired growth and weight gain, efficient feeding management is needed when rearing fish in this particular type of system using sweet flour as carbon source for biofloc production.
References
APHA (American Public Health Association), American Water Works Association, and Water Pollution Control Association (1989) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington, DC
Asaduzzaman M, Wahab MA, Verdegem MCJ, Huque S, Salam MA, Azim ME (2008) C/N ratio control and substrate addition for periphyton development jointly enhance freshwater prawn Macrobrachium rosenbergii production in ponds. Aquaculture 280:117–123
Avnimelech Y (1999) Carbon and nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235
Avnimelech Y (2006) Bio-filters: the need for an new comprehensive approach. Aquac Eng 34:172–178
Azim ME, Little DC (2008) The biofloc technology (BFT) in indoor tanks: water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 283:29–35
Ballester ELC, Abreu PC, Cavalli RO et al (2010) Effect of practical diets with different protein levels on the performance of Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system. Aquac Nutr 16:163–172
Baloi M, Arantes R, Schveitzer R, Magnotti C, Vinatea L (2013) Performance of Pacific white shrimp Litopenaeus vannamei raised in biofloc systems with varying levels of light exposure. Aquacult Eng 52:39–44
Caipang CMA, Choo HX, Bai Z, Huang H, Lay-Yag CM, Lim J (2015) Small-scale production of biofloc using various carbon sources for the freshwater culture of tilapia, Oreochromis sp. ABAH Bioflux 7:103–111
Choo HX, Caipang CMA (2015) Biofloc technology (BFT) and its application towards improved production in freshwater tilapia culture. AACL Bioflux 8:362–366
Correia ES, Wilkenfeld JS, Morris TC, Wei L, Prangnell DI, Samocha TM (2014) Intensive nursery production of the Pacific white shrimp Litopenaeus vannamei using two commercial feeds with high and low protein content in a biofloc-dominated system. Aquacult Eng 59:48–54
Crab R (2010) Bioflocs technology: an integrated system for the removal of nutrients and simultaneous production of feed in aquaculture. PhD thesis, Ghent University
Crab R, Avnimelech Y, Defoirdt T, Bossier P, Verstraete W (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14
Crab R, Kochva M, Verstraete W, Avnimelech Y (2009) Bio-flocs technology application in over-wintering of tilapia. Aquacult Eng 40:105–112
Crab R, Chielens B, Wille M, Bossier P, Verstraete W (2010) The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquac Eng 41:559–567
Crab R, Defoirdt T, Bossier P, Verstraete W (2012) Biofloc technology in aquaculture: beneficial effects and future challenges. Aquaculture 365–357:351–356
De Schryver P, Verstraete W (2009) Nitrogen removal from aquaculture pond water by heterotrophic nitrogen assimilation in lab-scale sequencing batch reactors. Bioresour Technol 100:1162–1167
De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W (2008) The basics of bio-flocs technology: the added value for aquaculture. Aquaculture 277:125–137
Ekasari J, Crab R, Verstraete W (2010) Primary nutritional content of bio-flocs cultured with different organic carbon sources and salinity. HAYATI J Biosci 17:125–130
Ekasari J, Angela D, Waluyo SH, Bachtiar T, Surawidjaja EH, Bosier P, De Schryver P (2014) The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 426–427:105–111
Hargreaves JA (2006) Photosynthetic suspended-growth systems in aquaculture. Aquac Eng 34:344–363
Little DC, Murray JF, Azim ME, LeschenW Grady K, Young J, Watterson A (2008) Warm-water fish production in the UK: limits to green growth? Trends Food Sci Technol 19:255–264
Piedrahita RH (2003) Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 226:35–44
Schveitzer R, Arantes R, Costodio PFS, do Espirito Santo CM, Arana LV, Seiffert WQ, Andreatta ER (2013) Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacult Eng 56:59–70
Wang G, Yu E, Xie J, Yu D, Li Z, Luo W, Qiu L, Zheng Z (2015) Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture 443:98–104
Yang SP, Qiu DQ (2006) Water quality in the high-density shrimp culturing ponds. Fisheries Sci 25:459–462
Acknowledgments
The data included in this paper are part of the Major Student’s Project (Optimization Studies of Biofloc Technology (BFT) in Freshwater Tilapia Culture: Effects on Water Quality and Fish Growth) of H.X. Choo. The authors gratefully acknowledge the funding support provided by the School of Applied Science, Temasek Polytechnic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of the co-authors, I declare that there is no conflict of interest regarding the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Caipang, C.M.A., Choo, H.X., Bai, Z. et al. Viability of sweet potato flour as carbon source for the production of biofloc in freshwater culture of tilapia, Oreochromis sp.. Int Aquat Res 7, 329–336 (2015). https://doi.org/10.1007/s40071-015-0117-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40071-015-0117-7