Abstract
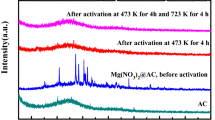
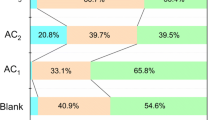
To obtain a material that adsorbs nitrogen oxide (NOx) gas with high efficiency, we used sulfuric acid (H2SO4)-treated activated carbon (AC). The proposed H2SO4-treated AC was treated by dipping and spray coating in a H2SO4 solution. For structural analysis, scanning electron microscopy and Brunauer–Emmett–Teller measurements were performed on general and H2SO4-treated ACs. The composition of the atoms on the surface and inner pore structure of both ACs was confirmed through energy dispersive X-ray spectroscopy measurements. From the structural analysis, it was confirmed that the structure of H2SO4-treated AC was similar to that of general AC, indicating that it was not affected by the H2SO4 solution. There was no significant difference in the inner pore structures of general and H2SO4-treated ACs through specific surface area analysis, which indicates that the physical adsorption of NOx gas for the H2SO4-treated AC is similar to that of general AC. Based on the atomic composition analysis, it was confirmed that sulfur ions were largely present on the inner pore structure than the surface of H2SO4-treated AC. These results indicate that the chemical adsorption of sulfur ions and NOx gas in the inner pore structure occurred rather than physical adsorption. The results of this study are expected to be applicable to the field of materials that remove harmful gases.





Similar content being viewed by others
References
J.F. Currie, A. Essalik, J.-C. Marusic, Sens. Actuators B 59, 235 (1999)
H. Ritchie, M. Roser, Our World in Data (2017). https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions
K. Gillingham, J.H. Stock, J. Econ. Perspect 32, 53 (2018)
S.J. Davis, K. Caldeira, H.D. Matthews, Science 329, 1330 (2010)
S. Sillman, Atmos. Environ. 33, 1821 (1999)
S.-B. Lee, G.-N. Bae, Y.-M. Lee, K.-C. Moon, M. Choi, Aerosol Air Qual. Res. 10, 540 (2010)
H.J. Seo, R.H. Jeong, J.-H. Boo, J. Song, J.-H. Boo, Appl. Sci. Converg. Technol. 26, 2180 (2017)
C. Wang, Y. Liu, S. Zheng, A. Jiang, Energy 153, 149 (2018)
J.V. Caneghem, J.D. Greef, C. Block, C. Vandecasteele, J. Clean. Prod. 112, 4452 (2016)
H. Yu, W. Dai, G. Qian, X. Gong, D. Zhou, X. Li, Z. Zhou, Nanomaterials 10, 897 (2020)
M. Xu, Y. Bao, K. Wu, T. Xia, H.L. Clack, H. Shi, V.C. Li, Constr. Build. Mater. 221, 375 (2019)
Y.-C. Chiang, P.-C. Chiang, C.-P. Huang, Carbon 39, 523 (2001)
B. Kasprzyk-Hordern, Adv. Colloid Interface Sci. 110, 19 (2004)
J. Li, H. Chang, L. Ma, J. Hao, R.T. Yang, Catal. Today 175, 147 (2011)
V. Valtchev, L. Tosheva, Chem. Rev. 113, 6734 (2013)
O.I. Polivaevm, A.N. Larionov, I.A. Spitsyn, A.V. Bozhko, L.K. Gorban, I.O.P. Conf, Ser. Mater. Sci. Eng. 632, 012027 (2019)
T. Lim, Y. La, O.S. Jeon, S.Y. Park, Y.J. Yoo, K.-H. Yang, J. Korean Phys. Soc. 77, 790 (2020)
A.M. Rubel, J.M. Stencel, Energy Fuels 10, 704 (1996)
K. Silas, W.A.W.A.K. Ghani, T.S.Y. Choong, U. Rashid, Catal. Rev. 61, 134 (2019)
C.-C. Huang, H.-S. Li, C.-H. Chen, J. Hazard. Mater. 159, 523 (2008)
S. Brunauer, P.H. Emmett, E. Teller, J. Am. Chem. Soc. 60, 309 (1938)
E.P. Barrett, L.G. Joyner, P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951)
C.L. Cllfton, N. Altstein, R.E. Hule, Environ. Sci. Technol. 22, 586–589 (1988)
M.A. Siddiqi, J. Petersen, Ind. Eng. Chem. Res. 40, 2116–2127 (2001)
Acknowledgements
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20181110200070); Materials, Components & Equipment Research Program funded by Gyeonggi Province.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, Y.P., Lee, Sh., Song, J.Y. et al. Reinforced nitrogen oxide gas adsorption by sulfur ionic treatment. J. Korean Phys. Soc. 79, 1051–1056 (2021). https://doi.org/10.1007/s40042-021-00317-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40042-021-00317-6