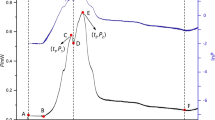
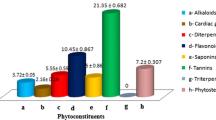
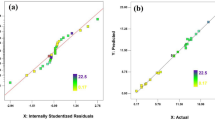
Abstract
Multidrug resistance (MDR) pathogens Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species are the main causative agents of infections in a majority of both developed and underdeveloped countries hospitals. Medicinal plant-based antimicrobial agents can be used as an alternate source to treat microbial infection caused by antibiotic-resistant microorganisms. The present study was designed to evaluate antimicrobial properties of Adhatoda vasica plant leave methanolic extract against three MDR pathogens isolated from local hospital and identified using 16S rRNA, viz. S. aureus, P. aeruginosa, and A. pitti. In the present study, Soxhlet extraction methodology was used to optimize the bioactive compounds from A. vasica leaves. The ideal extraction parameters for the highest antibacterial activity in terms of zone of inhibition were duration (30 min), temperature (70 °C), and solid–liquid ratio (30 g/L) utilizing response surface methodology and central composite design (mm). Analysis of antimicrobial activity was performed against three MDR pathogens, namely S. aureus, P. aeruginosa, and A. pitti, by using disk diffusion assay. GC–MS profiling revealed the presence of active phytochemicals such as 6,9,12-octadecatrienoic acid, phenylmethyl ester, and phytol known for pharmacological importance. From the present work, it can be concluded that A. vasica extracts could be a viable source to combat infection caused by MDR pathogens in both community and hospital settings.







Similar content being viewed by others
Code Availability
Not applicable.
Abbreviations
- MDR:
-
Multidrug resistance
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- VRE:
-
Vancomycin-resistant Enterococcus faecium
- PRSP:
-
Penicillin-resistant Streptococcus pneumoniae
- ARB:
-
Antimicrobial resistance bacteria
- AST:
-
Antibiotic sensitivity test
- MHA:
-
Mueller–Hinton agar
References
Shariati A, Dadashi M, Chegini Z, van Belkum A, Mirzaii M, Khoramrooz SS, Darban-Sarokhalil D (2020) The global prevalence of daptomycin, tigecycline, quinupristin/dalfopristin, and linezolid-resistant staphylococcus aureus and coagulase–negative staphylococci strains: a systematic review and meta-analysis. Antimicrob Resist Infect Control 9:1–20
Mishra, M., Patole, S., and Mohapatra, H. (2021). Nanoparticles: powerful tool to mitigate antibiotic resistance. Sustain Agric Rev 49:171–204.
Gorlenko CL, Kiselev HY, Budanova EV, Zamyatnin AA, Ikryannikova LN (2020) Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: new heroes or worse clones of antibiotics? Antibiotics 9:170
Shukla S, Hegde S, Kumar A, Chaudhary G, Tewari S, Upreti D, Pal M (2017) Chemical composition and antibacterial activity of essential oil from leaves of Justicia adhatoda against methicillian resistant and sensitive strain along with their clinical isolates. J Essent Oil-Bear Plants 20:116–122
Kumari N, Akhtar J, Ahmad M, Badruddeen, and Khan, M.I. (2021) An outline on vasicine, its ethnomedical and nanoformulation approach. J Biol Act Prod Nat 11:42–59
Raghuvanshi D, Dhalaria R, Sharma A, Kumar D, Kumar H, Valis M, Kuča K, Verma R, Puri S (2021) Ethnomedicinal plants traditionally used for the treatment of jaundice (Icterus) in Himachal Pradesh in Western Himalaya—A Review. Plants 10:232
Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Parveen R, Ahmad M (2021) Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Front Pharmacol 11:2470
Panigrahi J, Gantait S, Patel IC (2017) Concurrent production and relative quantification of vasicinone from in vivo and in vitro plant parts of Malabar nut (Adhatoda vasica Nees). 3 Biotech 7, 1–8.
Nikhitha J, Swathy K, Chandran RP (2021) In vitro anticancer activity of ethanol extract of Adhatoda vasica Nees on human ovarian cancer cell lines. J Genet Eng Biotechnol 19:1–9
Abu Bakar FI, Abu Bakar MF, Abdullah N, Endrini S, Fatmawati S (2020) Optimization of extraction conditions of phytochemical compounds and anti-gout activity of Euphorbia hirta L.(ara tanah) using response surface methodology and liquid chromatography-mass spectrometry (LC-MS) analysis. Evid-Based Complementary Altern Med
Arora D, Mahajan H (2018) In vitro evaluation and statistical optimization of antimicrobial activity of Prunus cerasoides stem bark. Appl Biochem Biotechnol 184:821–837
Dutescu IA, Hillier SA (2021) Encouraging the development of new antibiotics: Are financial incentives the right way forward? A systematic review and case study. Infect Drug Resist 14:415
Abiola, I. (2019). Investigating the mechanisms of antimicrobial resistance and virulence of bacteria isolated from hospital environments. (University of Ghana).
Bauer A (1966) Antibiotic susceptibility testing by a standardized single disc method. Am J clin pathol 45:149–158
Sambrook, J., and Russell, D.W. (2006). Purification of nucleic acids by extraction with phenol: chloroform. Cold Spring Harbor Protocols 2006, pdb. prot4455.
Ojha SK, Singh PK, Mishra S, Pattnaik R, Dixit S, Verma SK (2020) Response surface methodology based optimization and scale-up production of amylase from a novel bacterial strain, Bacillus aryabhattai KIIT BE-1. Biotechnol Rep 27:e00506
Dwibedi V, Rath SK, Prakash R, Saxena S (2021) Response surface statistical optimization of fermentation parameters for resveratrol production by the endophytic fungus Arcopilus aureus and its tyrosinase inhibitory activity. Biotechnol Rep 43:627–644
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity. J Pharm Anal 6:71–79
Institute, C.L.S. (2017). Performance standards for antimicrobial susceptibility testing (2017) CLSI approved standards CLSI M100-S23. (CLSI Wayne^ ePA PA).
Performance C (2016) Standards for antimicrobial susceptibility testing, CLSI supplement M100S. Clinical and Laboratory Standards Institute, Wayne
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Ungureanu A, Zlatian O, Mitroi G, Drocaş A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii VN (2017) Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol Med Rep 16:8771–8780
Mishra MP, Sarangi R, Padhy RN (2016) Prevalence of multidrug resistant uropathogenic bacteria in pediatric patients of a tertiary care hospital in eastern India. J Infect Public Health 9:308–314
Dan M, Yehui W, Qingling M, Jun Q, Xingxing Z, Shuai M, Kuojun C, Jinsheng Z, Zibing C, Zaichao Z (2019) Antimicrobial resistance, virulence gene profile and molecular typing of Staphylococcus aureus isolates from dairy cows in Xinjiang Province, northwest China. J Glob Antimicrob Resist 16:98–104
Srivastava R, Agarwal J, Srivastava S, Kumar M, Singh M (2014) Multidrug resistant gram-negative bacilli from neonatal septicaemia at a tertiary care centre in North India: a phenotypic and genotypic study. Indian J Med Microbiol 32:97
Ayoub Moubareck C, Hammoudi Halat D (2020) Insights into Acinetobacter baumannii: a review of microbiological, virulence, and resistance traits in a threatening nosocomial pathogen. Antibiotics 9:119
Shakibaie MR, Adeli S, Salehi MH (2012) Antibiotic resistance patterns and extended-spectrum β-lactamase production among Acinetobacter spp. isolated from an intensive care Unit of a hospital in Kerman. Iran Antimicrob Resist Infect Control 1:1–8
Ichioka S, Nakatsuka T, Ohura N, Sato Y, Harii K (2001) Clinical use of amrinone (a selective phosphodiesterase III inhibitor) in reconstructive surgery. Plast Reconstr Surg 108:1931–1937
Bharathy V, Sumathy BM, Uthayakumari F (2012) Determination of phytocomponents by GC-MS in leaves of Jatropha gossypifolia L. Sci res report 2:286–290
Acknowledgements
The authors acknowledge the Chandigarh University for providing facilities.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MJ and VD conceived the idea, designed the experiments, carried out the experiments, and wrote the manuscript. SR and TM helped in data analysis. SK supervised the work and edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of Interest.
Consent for Publication
Not applicable.
Consent on Studies with Human and Animal Subjects
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance’ Statement
The plant extract was evaluated for its antimicrobial potential against clinical isolates. The clinical isolates were isolated which showed antimicrobial resistance against three or more antibiotics. The plant extract concentrations were optimized with the help of RSM. One of the aspects was to explore the potential of plant extract against antibiotic-resistant bacteria and to evaluate the presence of various phytochemical in the plant extract.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, M., Dwibedi, V., Rath, S.K. et al. Process Optimization of Antimicrobial Activity of Adhatoda vasica Against MDR Pathogens Using Response Surface Methodology. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 94, 47–58 (2024). https://doi.org/10.1007/s40011-023-01504-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-023-01504-0