Abstract
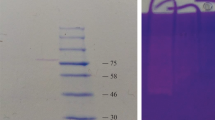
We isolated a bacterium (BBAU-19) producing alkaline protease from the saline soil of Uttar Pradesh, India. The isolate identified through 16S rRNA gene analysis was Bacillus pseudofirmus. The enzyme production of BBAU-19 was optimum at pH 9 and a temperature of 40 °C. The ammonium sulfate salt partially purified the crude enzyme. The filtered enzyme was analyzed under ion-exchange chromatography through a DEAE-cellulose column. The partially purified enzyme (PPE) activity increased up to ~ 1.92-fold compared to crude protease, and the recovery of protease enzyme was 54.60%. The molecular weight of the PPE was determined to be 42 kDa. The optimum temperature and pH of the PPE were 40 °C and 10.0, respectively. Ca++ and Mg++ ions enhanced the enzyme activity, while Cu++and Na+ ions suppressed it. The presence of ethylenediaminetetraacetic acid completely inhibited the enzyme activity. The findings of this study suggest that the enzyme is a Metallo-type protease in nature. We evaluated the efficiency of alkaline protease by testing its ability to remove stains of the cloth stained with Bradford reagent. The compatibility test with detergents enhanced the commercial use of this enzyme. The PPE (partially purified enzyme) was compatible with Ghadi and Nirma detergents, and the de-staining was maximum with 500 ml of the PPE.
Graphical Abstract
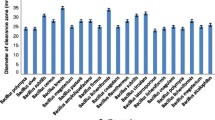
A Screening of the bacterial isolate BBAU-19, B Enzyme production, C Biochemical assay and enzyme activity determination of the bacterial isolate BBAU-19; D Purification and detergent formulation; BSA-soaked cloths were washed with—(DA) only water, (DB) local laundry detergents, (DC) local laundry detergents containing 250 ml of alkaline protease and (DD) local laundry detergents containing 500-ml alkaline protease followed by Bradford staining [Cloths washed with only water (DA) displayed maximum blue color, while cloths washed with detergent containing 500 ml of the enzyme (DD) displayed minimum blue color after Bradford staining]







Similar content being viewed by others
References
Razzaq A, Shamsi S, Ali A, Ali Q, Sajjad M, Malik A, Ashraf M (2019) Microbial proteases applications. Front Bioeng Biotechnol 7:110. https://doi.org/10.3389/fbioe.2019.00110
Sharma M, Gat Y, Arya S, Kumar V, Kumar A (2019) Review on microbial alkaline protease: an essential tool for various industrial approaches. Ind Biotechnol 15(2):69–78. https://doi.org/10.1089/ind.2018.0032
Dorra G, Ines K, Imen SB, Laurent C, Sana A, Olfa T, Pascal C, Thierry J, Ferid L (2018) Purification and characterization of a novel high molecular weight alkaline protease produced by an endophytic Bacillus halotolerans strain CT2. Int J Biol Macromol 111:342–351. https://doi.org/10.1016/j.ijbiomac.2018.01.024
Cavello I, Bezus B, Cavalitto S (2021) The keratinolytic bacteria Bacillus cytotoxicus as a source of novel proteases and feather protein hydrolysates with antioxidant activities. J Genet Eng Biotechnol 19:107. https://doi.org/10.1186/s43141-021-00207-1
Sundus H, Mukhtar H, Nawaz A (2016) Industrial applications and production sources of serine alkaline proteases: a review. J Bacteriol Mycol 3(1):1–4. https://doi.org/10.15406/jbmoa.2016.03.00051
Cui H, Wang L, Yu Y (2015) Production and characterization of alkaline protease from a high yielding and moderately halophilic strain of SD11 MARINE BACteria. J Chem. https://doi.org/10.1155/2015/798304
Chatterjee J, Giri S, Maity S, Sinha A, Ranjan A, Rajshekhar et al (2015) Production and characterization of thermostable alkaline protease of Bacillus subtilis (ATCC 6633) from optimized solid-state fermentation. Biotechnol Appl Biochem 62:709–718. https://doi.org/10.1002/bab.1309
Ibrahim ASS, Al-Salamah AA, Elbadawi YB, El-Tayab MA, Ibrahim SSS (2015) Production of extracellular alkaline protease halotolerentalkaliphilic Bacillus sp. NPST-AK15 isolated from hyper saline soda lakes. Electron J Biotechnol 18:236–243. https://doi.org/10.1016/j.ejbt.2015.04.001
Verma J, Pandey S (2019) Characterization of partially purified alkaline protease secreted by halophilic bacterium Citricoccus sp. isolated from agricultural soil of northern India. Biocatal Agric Biotechnol 17:605–612. https://doi.org/10.1016/j.bcab.2019.01.020
Cheng K, Lu FP, Li M, Liu LL, Liang XM (2010) Purification and biochemical characterization of a serine alkaline protease TC4 from a new isolated Bacillus alcalophilus TCCC11004 in detergent formulations. Afr J Biotechnol 9:4942–4953
Tsuchida O, Yamagata Y, Ishizuka J (1986) An Alkaline proteinase an alkalophilic Bacillus sp. Curr Microbiol 14:7–12. https://doi.org/10.1007/BF01568094
Lowry OH, Rosebrough NI, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Upadhyay SK, Chandrashekar K (2012) Interaction of salivary and Midgut Proteins of Helicoverpa armigera with soybean Trypsin Inhibitors. Protein J 31:259–115. https://doi.org/10.1007/s10930-012-9402-0
Rao CS, Sathish T, Ravichandra P, Prakasham R (2009) Characterization of thermo-and detergent stable serine protease from isolated Bacillus circulans and evaluation of eco-friendly applications. Process Biochem 44(3):262–268. https://doi.org/10.1016/j.procbio.2008.10.022
Mothe T, Sultanpuram VR (2016) Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus. 3 Biotech 6:53. https://doi.org/10.1007/s13205-016-0377-y
Bradford MM (1976) A rapid and sensitive method for the quantitation of microorganism quantities of protein utilizing the principle of protein–dye binding. Ann Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Sen S, Veeranki VD, Mandal B (2009) Effect of physical parameters, carbon and nitrogen sources onthe production of alkaline protease from a newly isolated Bacillus pseudofirmus SVB1. Ann Microbiol 59(3):531–538
Gupta R, Beg OK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial application. Appl Microbiol Biotechnol 59:15–32. https://doi.org/10.1007/s00253-002-1142-1
Saggu SK, Mishra PC (2017) Characterization of thermostable alkaline proteases from Bacillus infantis SKS1 isolated from garden soil. PLoS ONE 12(11):e0188724. https://doi.org/10.1371/journal.pone.0188724
Sepahy AA, Jabalameli L (2011) Effect of culture conditions on the production of an extracellular protease by Bacillus sp. Isolated from soil sample of Lavizan Jungle park. Enzyme Res 2011:1–7. https://doi.org/10.4061/2011/219628
Ahmetoglu N, Bekler FM, Acer O, Guven RG, Guven K (2015) Production, purification and characterization of thermostable metallo-protease from newly isolated Bacillus sp. KG5. Eurasia J Biosci 9:1–11. https://doi.org/10.5053/ejobios.2015.9.0.l
Vijayraghavan P, Lazarus S, Vincent SGP (2014) De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: Biosynthesis and properties. Saudi J Biol Sci 21(1):27–34. https://doi.org/10.1016/j.sjbs.2013.04.010
Iqbal A, Hakim A, Hossain MS, Rahman MR, Islam K, Azim MF, Ahmed J, Assaduzzaman M, Hoq MM, Azad AK (2018) Partial purification and characterization of serine protease produced through fermentation of organic municipal solid wastes by Serratia marcescens A3 and Pseudomonas putida A2. J Genet Eng Biotechnol 16:29–37. https://doi.org/10.1016/j.jgeb.2017.10.011
Waghmore S, Gurav AA, Mali SA, Nadaf NH, Jadhav DB, Sonawane KD (2015) Purification and characterization of novel organic solvent tolerant 98 k Da alkaline protease isolated from Stenotrophomonas maltophilia strain SK. Protein Expr Purif 107:1–6. https://doi.org/10.1016/j.pep.2014.11.002
Manavalan T, Manavalan A, Ramachandran S, Heese K (2020) Protease from Bacillus megaterium -TK1 for the detergent and leather industry. Biology 9:472. https://doi.org/10.3390/biology9120472
Saxena S, Verma J, Shikha MDR (2014) RAPD-PCR and 16S rDNA phylogenetic analysis of alkaline protease producing bacteria isolated from soil of India: identification and detection of genetic variability. J Genet Eng Biotechnol 12:27–35. https://doi.org/10.1016/j.jgeb.2014.03.001
Saggu SK, Gopaljee J, Mishra PC (2019) Enzymatic degradation of biofilm by metalloprotease from Microbacterium sp. Sks 10. Front Bioeng Biotechnol 7:192. https://doi.org/10.3389/fbioe.2019.00192
Harwood CR, Kikuchi Y (2022) The ins and outs of Bacillus proteases: activities, functions and commercial significance. FEMS Microbiol Rev FUAB046 46:1–20. https://doi.org/10.1093/femsre/fuab046
Arnaouteli S, Bamford NC, Stanley-Wall NR et al (2021) Bacillus subtilis biofilm formation and social interactions. Nat Rev Microbiol 19:600–614
Inbaraj S, Agrawal RK, Thomas P, Chaudhuri P, Verma A, Chaturvedi VK (2022) Isolation and characterization of vB_SenS_Ib_psk2 bacteriophage against drug resistant Salmonella enterica serovar Kentucky. Res Sq. https://doi.org/10.21203/rs.3.rs-1900211/v1
Acknowledgements
This work was facilitated by the Department of Biotechnology from Babasaheb Bhimrao Ambedkar University, Lucknow 226025 (India), and the authors are thankful to execute the research work. The authors have no relevant financial or non-financial interests to disclose. Thus, there is no conflict of interest among the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We partially purified and characterized the bacteria Bacillus pseudofirmus (BBAU-19) from saline soil that produced alkaline protease. The optimum conditions for temperature and pH were 40 °C and 10, respectively. Ca++ and Mg++ ions activate the Metallo-type enzyme activity, whereas Cu ++and Na+ ions inhibit the enzyme activity. The enzyme compatibility was evaluated with two detergents (Ghadi and Nirma), and the identified enzyme was efficient in removing cloth stains.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, J., Pandey, S., Kumar, C. et al. A Detergent-Compatible Alkaline Metalloprotease from Bacillus pseudofirmus BBAU-19: Characterization and Application. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 93, 499–510 (2023). https://doi.org/10.1007/s40011-022-01436-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-022-01436-1