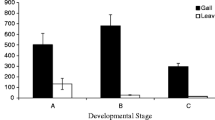
Abstract
Gall-inducing insects are highly specialized in modifying phenotypes in their hosts. Phytochemical manipulations in galling sites induce tissue growth and differentiation and also refurbish defense response in plant against herbivore infestation. Therefore, plant–herbivore interaction coevolves and gives rise to a chemical arms race by employing refined chemical defense and detoxification mechanisms in the plant. Under this contextual, we aimed to investigate how phytochemical gradients accumulate in galling sites than the non-galled tissue. Analyzing 18 phytochemicals from underdeveloped and developed foliar gall tissue morphs among three model plants, we report that phytochemical manipulation builds gradually from non-galled (non-infested) to underdeveloped gall (marginal infestation) and from underdeveloped to developed (high infestation) gall tissue. A complex chemical surge is played in galling tissue where the phytochemicals perform a dual role in promotion of tissue growth as well as in execution of endogenous defense.




Similar content being viewed by others
References
Arimura GI, Kost C, Boland W (2005) Herbivore-induced, indiretplandefences. Biochimica et Biophysica Acta Mol Cell Biol Lipids 1734(2):91–111. https://doi.org/10.1016/j.bbalip.2005.03.001
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology. Oxford: Oxford University Press, 421
Fatouros NE, Bukovinszkine’Kiss G, Kalkers LA, Gamborena RS, Dicke M, Hilker M (2005) Oviposition-induced plant cues: do they arrest Trichogramma wasps during host location? Entomologia Experimentalis et Applicata. 115(1):207–215. https://doi.org/10.1111/j.1570-7458.2005.00245.x
Amorim DO, Ferreira BG, Fleury G (2017) Plant potentialities determine anatomical and histochemical diversity in MikaniaglomerataSpreng. galls. Brazilian J Botany 40(2):517–527. https://doi.org/10.1007/s40415-016-0357-9
Harris MO, Pitzschke A (2020) Plants make galls to accommodate foreigners: some are friends, most are foes. New Phytol 225(5):1852–1872. https://doi.org/10.1111/nph.16340
Melnyk CW (2017) Connecting the plant vasculature to friend or foe. New Phytol 213(4):1611–1617. https://doi.org/10.1111/nph.14218
Sugio A, MacLean AM, Kingdom HN, Grieve VM, Manimekalai R, Hogenhout SA (2011) Diverse targets of phytoplasma effectors: from plant development to defense against insects. Annual Rev Phytopathol. 49:175–195. https://doi.org/10.1146/annurev-phyto-072910-09532
Fay PA, Hartnett DC, Knap AK (1993) Increased photosynthesis and water potentials in Silphiumintegrifolium galled by cynipid wasps. Oecologia 93(1):114–120
Erb M, MeldauS HGA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17(5):250–259. https://doi.org/10.1016/j.tplants.2012.01.003
Harris MO, Freeman TP, Rohfritsch O, Anderson KG, Payne SA, Moore JA (2006) Virulent Hessian fly (Diptera: Cecidomyiidae) larvae induce a nutritive tissue during compatible interactions with wheat. Ann Entomol Soc Am 99(2):305–316. https://doi.org/10.1603/0013-8746(2006)099[0305:VHFDCL]2.0.CO;2
Kang Z, Tang C, Zhao J, Cheng Y, Liu J, Guo J, Wan, X, Chen X (2017) Wheat-Pucciniastriiformis interactions. In: stripe rust pp 155–282
Nogueira RM, Costa EC, Silva JS, Isaias RMDS (2018) Structural and histochemical profile of Lopesia sp. Rübsaamen, 1908 pinnula galls on Mimosa tenuiflora (Willd.) Poir.in a Caatinga environment. Hoehnea 45(2):314–322. https://doi.org/10.1590/2236-8906-80/2017
Taper ML, Case TJ (1987) Interactions between oak tannins and parasite community structure: unexpected benefits of tannins to cynipid gall-wasps. Oecologia 71(2):254–261
Tooker JF, De Moraes CM (2009) A gall-inducing caterpillar species increases essential fatty acid content of its host plant without concomitant increases in phytohormone levels. Mol Plant-Microbe Interactions. 22(5):551–559. https://doi.org/10.1094/MPMI-22-5-0551
Tooker JF, De Moraes CM (2011) Feeding by a gall-inducing caterpillar species alters levels of indole-3-acetic and abscisic acid in Solidagoaltissima (Asteraceae) stems. Arthropod-Plant Interactions 5(2):115–124. https://doi.org/10.1007/s11829-010-9120-5
Davies PJ (2004) Plant hormones: biosynthesis, signal transduction, action! edn 3. Kluwer Academic Pub, Netherlands, p 750
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100(4):681–697. https://doi.org/10.1093/aob/mcm079
Oliveira DC, Isaias RMS, Fernandes GW, Ferreira BG, Carneir RGS, Fuzaro L (2016) Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol 84:103–113. https://doi.org/10.1016/j.jinsphys.2015.11.012
Rezende UC, Moreira ASFP, Kuster VC, de Oliveira DC (2018) Structural, histochemical and photosynthetic profiles of galls induced by Eugeniamyiadispar (Diptera: Cecidomyiidae) on the leaves of Eugenia uniflora (Myrtaceae).Revista de BiologíaTropical 66(4), 1469–1480. http://dx.doi.org/https://doi.org/10.15517/rbt.v66i4.32531
Passardi F, Longe D, Penel C, Dunand C (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65(13):1879–1893. https://doi.org/10.1016/j.phytochem.2004.06.023
Arimura GI, Kost C, Boland W (2005) Herbivore-induced, indirect plandefences. Biochimica et BiophysicaActa -Mol Cell Biol Lipids 1734(2):91–111. https://doi.org/10.1016/j.bbalip.2005.03.001
Turlings TC, McCall PJ, Alborn HT, Tumlinson JH (1993) An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J Chem Ecol 19(3):411–425
Diezel C, von Dahl GCC, Baldwin IT (2009) Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 150(3):1576–1586. https://doi.org/10.1104/pp.109.139550
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annual Rev Entomol 49(1):175–192. https://doi.org/10.1146/annurev.ento.49.061802.123236
Harris MO, Pitzschke A (2020) Plants make galls to accommodate foreigners: some are friends, most are foes. New Phytol 225(5):1852–1872. https://doi.org/10.1111/nph.16340
Ghosh D (2006) Bark is the hallmark. Resonance 11(3):41–50
Susy A, Switi R, Dhara G (2013) Anatomy and ontogenesis of foliar galls induced by Odinadiplosisodinae (Diptera: Cecidomyiidae) on Lanneacoramandelica (Anacardiaceae). Actaentomologicaserbica 18(1/2):161–175
Devaraj KB, Gowda LR, Prakash V (2008) An unusual thermostable aspartic protease from the latex of Ficus racemosa (L.). Phytochemistry 69(3):647–655. https://doi.org/10.1016/j.phytochem.2007.09.003
Tetgure SR, Borse AU, Sankapal BR, Garole VJ, Garole DJ (2015) Green biochemistry approach for synthesis of silver and gold nanoparticles using Ficus racemosa latex and their pH-dependent binding study with different amino acids using UV/Vis absorption spectroscopy. Amino Acids 47(4):757–765. https://doi.org/10.1007/s00726-014-1906-9
Azarkan M, Wintjens R, Looze Y, Baeyens-Volant D (2004) Detection of three wound-induced proteins in papaya latex. Phytochemistry 65:525–534. https://doi.org/10.1016/j.phytochem.2003.12.006
Price PW, Clancy KM (1986) Interactions among three trophic levels: gall size and parasitoid attack. Ecology 67(6):1593–1600. https://doi.org/10.2307/1939090
Rehill BJ, Schultz JC (2001) Hormaphishamamelidis and gall size: a test of the plant vigor hypothesis. Oikos 95(1):94–104. https://doi.org/10.1034/j.1600-0706.2001.950111.x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35(5):785–791. https://doi.org/10.1093/oxfordjournals.pcp.a078658
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Malick CP, Singh MB (1980) Phenolics. Kalyani Publishers, New Delhi, Plant enzymology and histoenzymology, p 286
Hatfield RD, Jung HJG, Ralph J, Buxton DR, Weimer PJ (1994) A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. J Sci Food Agric 65(1):51–58. https://doi.org/10.1002/jsfa.2740650109
Yamaguchi H, Tanaka H, Hasegawa M, Tokuda M, Asami T, Suzuki Y (2012) Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 196(2):586–595. https://doi.org/10.1111/j.1469-8137.2012.04264.x
Tanaka Y, Okada K, Asami T, Suzuki Y (2013) Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci Biotechnol Biochem. 77(9):1942–1948. https://doi.org/10.1271/bbb.130406
Silva ÉAS, Saboia G, Jorge NC, Hoffmann C, dos Santos Isaias RM, Soares GL, Zini CA (2017) Development of a HS-SPME-GC/MS protocol assisted by chemometric tools to study herbivore-induced volatiles in Myrciasplendens. Talanta 175:9–20. https://doi.org/10.1016/j.talanta.2017.06.063
Takahashi K, Akaike T, Sato K, Mori K, Maeda H (1993) Superoxide anion generation by Pacific oyster (Crassostreagigas) hemocytes: identification by electron spin resonance spintrapping and chemiluminescence analysis. Comparative Biochem Physiol Part B: Comparative Biochem 105(1):32–41
Kaur G, Hamid H, Ali A, Athar Alam MS., M, (2004) Antiinflammatory evaluation of alcoholic extract of galls of Quercusinfectoria. J Ethnopharmacol. 90(2–3):285–292. https://doi.org/10.1007/s11738-014-1528-6
Lipetz J, Galston AW (1959) Indole acetic acid oxidase and peroxidase activities in normal and crown gall tissue cultures of Parthenocissustricuspidata. Am J Bot 46(3):193–196. https://doi.org/10.1002/j.1537-2197.1959.tb07003.x
Isaias RMS, Oliveira DC, Moreira ASFP, Soares GLG, Carneiro RGS (2015) The imbalance of redox homeostasis in arthropod-induced plant galls: mechanisms of stress generation and dissipation
Xu J, Duan X, Yang J, Beeching JR, Zhang P (2013) Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 161(3):1517–1528. https://doi.org/10.1104/pp.112.212803
Donaghy L, Kraffe E, Le Goïc N, Lambert C, Volety AK, Soudant P (2012) Reactive oxygen species in unstimulatedhemocytes of the Pacific oyster Crassostreagigas: a mitochondrial involvement. PLoS ONE 7(10):46594. https://doi.org/10.1371/journal.pone.0046594
Son YO, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee JC, Shi X (2012) Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3β/β-catenin signaling. Toxicol Appl Pharmacol 264(2):153–160. https://doi.org/10.1016/j.taap.2012.07.028
Mukherjee S, Ray M, Dutta MK, Acharya A, Mukhopadhya SK, Ray S (2015) Morphological alteration, lysosomal membrane fragility and apoptosis of the cells of Indian fresh water sponge exposed to washing soda (sodium carbonate). Ecotoxicol Environ Saf 122:331–342. https://doi.org/10.1016/j.ecoenv.2015.08.011
Hartley SE, Lawton JH (1992) Host-plant manipulation by gall-insects: a test of the nutrition hypothesis. J Anim Ecol. https://doi.org/10.2307/5514
Weis AE, Walton R, Crego CL (1988) Reactive plant tissue sites and the population biology of gall makers. Annu Rev Entomol 33:467–486. https://doi.org/10.1146/annurev.en.33.010188.002343
Motta LB, Kraus JE, Salatino A, Salatino ML (2005) Distribution of metabolites in galled and non-galled foliar tissues of Tibouchinapulchra. Biochem Syst Ecol 33(10):971–981. https://doi.org/10.1016/j.bse.2005.02.004
Abrahamson WG, McCrea KD (1986) Nutrient and biomass allocation in Solidagoaltissima: effects of two stem gall makers, fertilization, and ramet isolation. Oecologia 68:174–180
Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44:1–24. https://doi.org/10.1146/annurev-genet-102209-163500
Kunkler N, Brandl R, Brandle M (2013) Changes in Clonal poplar leaf chemistry caused by stem galls Alter Herbivory and leaf litter decomposition. PLoS ONE 8(11):79994. https://doi.org/10.1371/journal.pone.0079994
Dsouza MR, Ravishankar BE (2014) Nutritional sink formation in galls of Ficus glomerata Roxb. (Moraceae) by the insect Pauropsylladepressa (Psyllidae, Hemiptera). Tropical Ecol. 55(1):129–136
Carneiro RGS, Castro AC, Isaias RMS (2014) Unique histochemical gradients in a photosynthesis-deficient plant gall. S Afr J Bot 92:97–104. https://doi.org/10.1016/j.sajb.2014.02.011
Moura MZD, Soare GLG, dos Santos Isaias RM (2008) Species-specific changes in tissue morphogenesis induced by two arthropod leaf gallers in Lantana camara L.(Verbenaceae). Australian J Botany. 56(2):153–160. https://doi.org/10.1071/BT07131
Hartley SE (1998) The chemical composition of plant galls: are levels of nutrients and secondary compounds controlled by the gall-former? Oecologia 113:492–501
Dicke M, Van Poecke RM (2002) Signalling in plant-insect interactions: signal transduction in direct and indirect plant defence. Plant Signal Transduct 289:316
Elzen G (1983) Cytokinins and insect galls. Comp Biochem Physiol A Physiol 76(1):17–19. https://doi.org/10.1016/0300-9629(83)90286-4
Jameson PE (2000) Cytokinins and auxins in plant-pathogen interactions–An overview. Plant Growth Regul 32(2–3):369–380
Zhang CX, He MX, Cao Y, Liu J, Gao F, Wang WB, Ji KP, Shao SC, Wang Y (2015) Fungus-insect gall of Phlebopusportentosus. Mycologia 107(1):12–20. https://doi.org/10.3852/13-267
Anand A, Uppalapati SR, Ryu CM, Allen SN, Kang L, Tang Y, Mysore KS (2008) Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol 146(2):703–715. https://doi.org/10.1104/pp.107.111302
Giba Z, Todorović S, Grubišić D, Konjević R (1998) Occurrence and regulatory roles of superoxide anion radical and nitric oxide in plants. IugoslavicaPhysiologica et PharmacologicaActa 34:447–461
Schmelz EA, Alborn HT, Engelbert J, Tumlinson JH (2003) Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol 133(1):295–306. https://doi.org/10.1104/pp.103.024174
Zander M, La Camera S, Lamotte O, Métraux JP, Gatz C (2010) Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J. 61(2):200–210. https://doi.org/10.1111/j.1365-313X.2009.04044.x
Zucker WV (1982) How aphids choose leaves: the roles of phenolics in host selection by a galling aphid. Ecology 63(4):972–981. https://doi.org/10.2307/1937237
Pascual-Alvarado E, Cuevas-Reye P, Quesada M, Oyama K (2008) Interactions between galling insects and leaf-feeding insects: the role of plant phenolic compounds and their possible interference with herbivores. J Trop Ecol 24(3):329–336
Anand A, Uppalapati SR, Ryu CM, Allen SN, Kang L, Tang Y, Mysore KS (2008) Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol 146(2):703–715. https://doi.org/10.1104/pp.107.111302
Hool LC (2005) Reactive oxygen species in cardiac signalling–from mitochondria to plasma membrane ion channels. Proc Aust Physiol Soc 36:55–61
de Oliveira DC, Moreira ASFP, dos Santos Isaias RM (2014) Functional gradients in insect gall tissues: studies on Neotropical host plants. In: neotropical insect galls, pp 35–49
Liu X, Williams CE, Nemacheck JA, Wan H, Subramanyam S, Zheng C, Chen MS (2010) Reactive oxygen species are involved in plant defense against a gall midge. Plant Physiol 152(2):985–999. https://doi.org/10.1104/pp.109.15065
Baldacci-Cresp F, Behr M, Kohler A, Badalato N, Morreel K, Goeminne G, Mol A, de Almeida Engler J, Boerjan W, El Jaziri M, Baucher M (2020) Molecular changes concomitant with vascular system development in mature galls induced by root-knot nematodes in the model tree host Populustremula× P. alba. Int J Mol Sci 21(2):406. https://doi.org/10.3390/ijms21020406
Yabuta Y, Motoki T, Yoshimura K, Takeda T, Ishikawa T, Shigeoka S (2002) Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant J. 32(6):915–925. https://doi.org/10.1046/j.1365-313X.2002.01476.x
Eshwarappa RSB, Ramachandra YL, Subaramaihha SR, Subbaiah SGB, Austi RS, Dhananjaya BL (2015) Antioxidant activities of leaf galls extracts of Terminaliachebula (Gaertn.) Retz (Combretaceae). Acta Sci Pol Technol Aliment 14(2):97–105. https://doi.org/10.17306/J.AFS.2015.2.11
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Biol 48(1):251–275. https://doi.org/10.1146/annurev.arplant.48.1.251
Das S, DeMason DA, Ehlers JD, Close T, Roberts PA (2008) Histological characterization of root-knot nematode resistance in cowpea and its relation to reactive oxygen species modulation. J Exp Bot 59(6):1305–1313. https://doi.org/10.1093/jxb/ern036
Kombrink E, Schmelzer E (2001) The hypersensitive response and its role in local and systemic disease resistance. Eur J Plant Pathol 107(1):69–78
Grün S, Lindermayr C, Sell S, Durner J (2006) Nitric oxide and gene regulation in plants. J Exp Bot 57(3):507–516. https://doi.org/10.1093/jxb/erj053
Mur LA, Prats E, Pierr S, Hall MA, Hebelstrup KH (2013) Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front Plant Sci 4:215. https://doi.org/10.3389/fpls.2013.00215
Urquiaga INES, Leighton F (2000) Plant polyphenol antioxidants and oxidative stress. Biol Res. 33(2):55–64. https://doi.org/10.7584/JKTAPPI.2018.04.50.2.5
Konno K (2011) Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry 72(13):1510–1530. https://doi.org/10.1016/j.phytochem.2011.02.016
Forslund H, Wikström SA, Pavia H (2010) Higher resistance to herbivory in introduced compared to native populations of a seaweed. Oecologia 164(3):833–840. https://doi.org/10.1007/s00442-010-1767-1
Kruzmane D, Jankevic L, Ievinsh G (2002) Effect of regurgitant from Leptinotarsadecemlineata on wound responses in Solanumtuberosum and Phaseolus vulgaris. Physiologia Plantarum 115(4):577–584. https://doi.org/10.1034/j.1399-3054.2002.1150412.x
Baştaş KK (2015) Importance of reactive oxygen species in plants-pathogens interactions. Selcuk J Agric Food Sci 28(1):11–21
Samsone I, Andersone U, Ievinsh G (2012) Variable effect of arthropod-induced galls on photochemistry of photosynthesis, oxidative enzyme activity and ethylene production in tree leaf tissues. Environ Experiment Biol 10:15–26
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
Krens FA, Molendijk L, Wullem GJ, Schilperoort RA (1985) The role of bacterial attachment in the transformation of cell-wall-regenerating tobacco protoplasts by Agrobacterium tumefaciens. Planta 166(3):300–308
Ighodaro OM, Akinloye OA (2018) First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Acknowledgements
We are thankful to Head of the Department of Zoology, University of Calcutta, for providing necessary facilities and for conducting the experiments. We thankfully acknowledge Centre for Research in Nano-science and Nano-technology (CRNN) of University of Calcutta for ROS experiment facility and Toxicology Laboratory from University of Calcutta for antioxidant profiling assays. This research did not receive any specific grant or financial support from any funding agency elsewhere. There is no conflict of interest in publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement The paper describes data to understand how chemical adaptation is being played in gall-induced foliar tissues from three model plants. How the assemblage of different phytochemical gradients in gall-affected tissue modulates plant–herbivore survivorship and assists coevolution has been portrayed.
Rights and permissions
About this article
Cite this article
Roy, S., Mukherjee, A., Gautam, A. et al. Chemical Arms Race: Occurrence of Chemical Defense and Growth Regulatory Phytochemical Gradients in Insect-Induced Foliar Galls. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 92, 415–429 (2022). https://doi.org/10.1007/s40011-021-01322-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-021-01322-2