Abstract
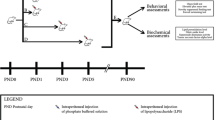
Preclinical and clinical studies have reported that psychiatric illness stems from exposure to infection during early life. Infection-induced increase in proinflammatory cytokines and further activation of the hypothalamic–pituitary–adrenal (HPA) axis are considered the main factors behind mental illness. The present study elucidated the impairment of HPA axis of female mice prenatally challenged with bacterial endotoxin lipopolysaccharide (LPS). The impairment was evaluated through detailed investigation of adrenal histopathology, the end target endocrine gland of HPA axis, plasma levels of pituitary adrenocorticotropic hormone (ACTH) and corticosterone (CORT). In view of the role of pituitary hormone prolactin (PRL) in modulation of HPA axis under infection, plasma level of PRL was also assessed. The evaluation was done at three different life stages of adulthood, i.e., postnatal day (PND) 85, PND 120 and PND 200. Distinct histopathological alterations in adrenal were revealed: distorted arrangement of zona fasciculata cells, localized cytoplasmic vacuolization and accumulation of lipid droplets were some of the disruptions of the adrenal cortex. Medulla showed distinct vascular congestion and disruptions of chromaffin cells with pyknotic nuclei. Plasma levels of ACTH and CORT were significantly elevated, but PRL was lowered. Bacterial endotoxin LPS might have interfered at different levels of hypothalamus, pituitary and directly on adrenal as well acting through its receptors to induce adrenotoxicity. An age-dependent HPA axis impairment was noted; the persistence of the adrenal toxicity was to a greater degree at PND 200 than PND 120 and PND 85 suggesting the reason for the susceptibility of old age to mental illness.







Similar content being viewed by others
References
Meltzer-Brody S, Larsen JT, Petersen L, Guintivano J, Florio AD, Miller WC, Sullivan PF, Munk-Olsen T (2018) Adverse life events increase risk for postpartum psychiatric episodes: a population-based epidemiologic study. Depress Anxiety 35(2):160–167. https://doi.org/10.1002/da.22697
Weinstock M (2017) Prenatal stressors in rodents: effects on behavior. Neurobiol Stress 6:3–13. https://doi.org/10.1016/j.ynstr.2016.08.004
Khandaker GM, Zimbron J, Lewis G, Jones PB (2013) Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 43(2):239–257. https://doi.org/10.1017/S0033291712000736
Boksa P (2010) Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 24(6):881–897. https://doi.org/10.1016/j.bbi.2010.03.005
Ronovsky M, Berger S, Zambon A, Reisinger SN, Horvath O, Pollak A, Lindtner C, Berger A, Pollak DD (2017) Maternal immune activation transgenerationally modulates maternal care and offspring depression-like behavior. Brain Behav Immun 63:127–136. https://doi.org/10.1016/j.bbi.2016.10.016
Kumar V (2019) Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. https://doi.org/10.1016/j.jneuroim.2019.03.012
Kanczkowski W, Chatzigeorgiou A, Samus M, Tran N, Zacharowski K, Chavakis T, Bornstein SR (2013) Characterization of the LPS-induced inflammation of the adrenal gland in mice. Mol Cell Endocrinol 371(1–2):228–235. https://doi.org/10.1016/j.mce.2012.12.020
Kentner AC, Pittman QJ (2010) Minireview: early-life programming by inflammation of the neuroendocrine system. Endocrinology 151(10):4602–4606. https://doi.org/10.1210/en.2010-0583
Haddad JJ, Saadé NE, Safieh-Garabedian B (2002) Cytokines and neuro–immune–endocrine interactions: a role for the hypothalamic–pituitary–adrenal revolving axis. J Neuroimmunol 133(1–2):1–19. https://doi.org/10.1016/S0165-5728(02)00357-0
Dahlgren J, Samuelsson AM, Jansson T, Holmäng A (2006) Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res 60(2):147. https://doi.org/10.1203/01.pdr.0000230026.74139.18
Shanks N, Larocque S, Meaney MJ (1995) Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci 15(1):376–384. https://doi.org/10.1523/JNEUROSCI.15-01-00376.1995
Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL (2000) Early-life exposure to endotoxin alters hypothalamic–pituitary–adrenal function and predisposition to inflammation. Proc Natl Acad Sci 97(10):5645–5650. https://doi.org/10.1073/pnas.090571897
Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A (2002) Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur J Endocrinol 146(2):251–260. https://doi.org/10.1530/eje.0.1460251
Basta-Kaim A, Budziszewska B, Leśkiewicz M, Fijał K, Regulska M, Kubera M, Wędzony K, Lasoń W (2011) Hyperactivity of the hypothalamus–pituitary–adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: effects of antipsychotic drugs. Eur J Pharmacol 650:586–595. https://doi.org/10.1016/j.ejphar.2010.09.083
Enayati M, Solati J, Hosseini MH, Shahi HR, Saki G, Salari AA (2012) Maternal infection during late pregnancy increases anxiety-and depression-like behaviors with increasing age in male offspring. Brain Res Bull 87(2–3):295–302. https://doi.org/10.1016/j.brainresbull.2011.08.015
Martinez-Arguelles DB, Guichard T, Culty M, Zirkin BR, Papadopoulos V (2011) In utero exposure to the antiandrogen di-(2-ethylhexyl) phthalate decreases adrenal aldosterone production in the adult rat. Biol Reprod 85(1):51–61. https://doi.org/10.1095/biolreprod.110.089920
Medwid S, Guan H, Yang K (2016) Prenatal exposure to bisphenol a disrupts adrenal steroidogenesis in adult mouse offspring. Environ Toxicol Pharmacol 43:203–208. https://doi.org/10.1016/j.etap.2016.03.014
Ping J, Wang JF, Liu L, Yan YE, Liu F, Lei YY, Wang H (2014) Prenatal caffeine ingestion induces aberrant DNA methylation and histone acetylation of steroidogenic factor 1 and inhibits fetal adrenal steroidogenesis. Toxicology 321:53–61. https://doi.org/10.1016/j.tox.2014.03.011
He Z, Zhu C, Huang H, Liu L, Wang L, Chen L, Magdalou J, Wang H (2016) Prenatal caffeine exposure-induced adrenal developmental abnormality in male offspring rats and its possible intrauterine programming mechanisms. Toxicol Res 5(2):388–398. https://doi.org/10.1039/c5tx00265f
Yan YE, Liu L, Wang JF, Liu F, Li XH, Qin HQ, Wang H (2014) Prenatal nicotinic exposure suppresses fetal adrenal steroidogenesis via steroidogenic factor 1 (SF-1) deacetylation. Toxicol Appl Pharmacol 277(3):231–241. https://doi.org/10.1016/j.taap.2014.03.019
Huang H, He Z, Zhu C, Liu L, Kou H, Shen L, Wang H (2015) Prenatal ethanol exposure-induced adrenal developmental abnormality of male offspring rats and its possible intrauterine programming mechanisms. Toxicol Appl Pharmacol 288(1):84–94. https://doi.org/10.1016/j.taap.2015.07.005
Calejman CM, Astort F, Di Gruccio JM, Repetto EM, Mercau M, Giordanino E, Sanchez R, Pignataro O, Arias P, Cymeryng CB (2011) Lipopolysaccharide stimulates adrenal steroidogenesis in rodent cells by a NFκB-dependent mechanism involving COX-2 activation. Mol Cell Endocrinol 337(1–2):1–6. https://doi.org/10.1016/j.mce.2010.12.036
Held Hales K, Diemer T, Ginde S, Shankar BK, Roberts M, Bosmann HB, Hales DB (2000) Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology 141(11):4000–4012. https://doi.org/10.1210/endo.141.11.7780
Ferreira LG, Prevatto JP, Freitas HR, Reis RA, Silva PM, Martins MA, Faria RX, Carvalho VF (2019) Capsaicin inhibits lipopolysaccharide-induced adrenal steroidogenesis by raising intracellular calcium levels. Endocrine 64(1):169–175. https://doi.org/10.1007/s12020-019-01849-5
De Laurentiis A, Pisera D, Caruso C, Candolfi M, Mohn C, Rettori V, Seilicovich A (2002) Lipopolysaccharide-and tumor necrosis factor-α-induced changes in prolactin secretion and dopaminergic activity in the hypothalamic-pituitary axis. NeuroImmunoModulation 10(1):30–39. https://doi.org/10.1159/000064412
Hollis JH, Lightman SL, Lowry CA (2005) Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic–pituitary–adrenal axis and inhibition of prolactin secretion. J Endocrinol 184(2):393–406. https://doi.org/10.1677/joe.1.05839
Kumar U, Mohanty B (2015) Atypical antipsychotic paliperidone prevents behavioral deficits in mice prenatally challenged with bacterial endotoxin lipopolysaccharide. Eur J Pharmacol 747:181–189. https://doi.org/10.1016/j.ejphar.2014.09.011
Kaufman MH, Kaufman MH (1992) The atlas of mouse development, vol 428. Academic press, London
Mishra AC, Mohanty B (2010) Lactational exposure to atypical antipsychotic drugs disrupts the pituitary-testicular axis in mice neonates during post-natal development. J Psychopharmacol 24(7):1097–1104. https://doi.org/10.1177/0269881109348162
Hewagalamulage SD, Clarke IJ, Rao A, Henry BA (2016) Ewes with divergent cortisol responses to ACTH exhibit functional differences in the hypothalamo-pituitary-adrenal (HPA) axis. Endocrinology. 157(9):3540–3549. https://doi.org/10.1210/en.2016-1287
Bhaskar R, Mishra AK, Mohanty B (2017) Neonatal exposure to endocrine disrupting chemicals impairs learning behaviour by disrupting hippocampal organization in male Swiss Albino mice. Basic Clin Pharmacol Toxicol 121(1):44–52. https://doi.org/10.1111/bcpt.12767
Doosti MH, Bakhtiari A, Zare P, Amani M, Majidi N, Babri S, Salari AA (2013) Impacts of early intervention with fluoxetine following early neonatal immune activation on depression-like behaviors and body weight in mice. Prog Neuropsychopharmacol Biol Psychiatry 43:55–65. https://doi.org/10.1016/j.pnpbp.2012.12.003
Walker FR, Owens J, Ali S, Hodgson DM (2006) Individual differences in glucose homeostasis: do our early life interactions with bacteria matter? Brain Behav Immun 20(4):401–409. https://doi.org/10.1016/j.bbi.2005.11.004
Tkachenko IV, Jaaskelainen T, Jaaskelainen J, Palvimo JJ, Voutilainen R (2011) Interleukins 1α and 1β as regulators of steroidogenesis in human NCI-H295R adrenocortical cells. Steroids 76(10–11):1103–1115. https://doi.org/10.1016/j.steroids.2011.04.018
Olukole SG, Lanipekun DO, Ola-Davies EO, Oke BO (2019) Melatonin attenuates bisphenol a-induced toxicity of the adrenal gland of Wistar rats. Environ Sci Pollut Res 26(6):5971–5982. https://doi.org/10.1007/s11356-018-4024-5
Rosol TJ, Yarrington JT, Latendresse J, Capen CC (2001) Adrenal gland: structure, function, and mechanisms of toxicity. Toxicol Pathol 29(1):41–48. https://doi.org/10.1080/19262301301418847
Sayed MM (2016) Effect of prenatal exposure to nicotine/thiocyanate on the pituitary–adrenal axis of 1-month-old rat offspring. Egypt J Histol 39(4):307–316. https://doi.org/10.1097/01.EHX.0000512120.79296.60
Douglas SA, Sreenivasan D, Carman FH, Bunn SJ (2010) Cytokine interactions with adrenal medullary chromaffin cells. Cell Mol Neurobiol 30(8):1467–1475. https://doi.org/10.1007/s10571-010-9593-x
Duijne SV (2012) Stress-related changes in adrenal glands of stranded Harbour Porpoises (Phocoena phocoena) on the Dutch coast (Master’s thesis)
Acknowledgements
The research work was supported partially by a grant from the Indian Council of Medical Research (ICMR), India, Project No. 58/10/2010-BMS to Dr. Banalata Mohanty. Fellowship of University Grant Commission to Mrs. Preeti Gupta is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
This study suggests that early-life infection could have long-lasting impact to cause impairment of the hypothalamic–pituitary–adrenal axis which may lead to not only the diseases related to adrenal but also reflect in other diseases at later ages.
Rights and permissions
About this article
Cite this article
Gupta, P., Mohanty, B. Assessment of Adrenotoxicity Induced on Prenatal Exposure to Bacterial Endotoxin Lipopolysaccharide: an Age-Related Study in Mice. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 1035–1044 (2020). https://doi.org/10.1007/s40011-020-01167-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01167-1