Abstract
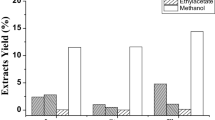
Pre-fractionation and contemporary spectroscopic analysis provides important clues with regard to synergistic and antagonistic effects as well as biological activities in complex multi-mixtures, especially natural products. Phytochemical contents of the Chrysophthalmum montanum root extracts were analyzed by gas chromatography–mass spectrometry and high performance liquid chromatography–time of flight/mass spectrometry. Furthermore, biological activity of root extract was investigated by antiproliferative efficiencies against human uterus carcinoma and rat brain tumor cells in vitro. The biological activity tests were carried out as dose-dependent assay using a multiwell spectrophotometer. 5-Fluorouracil was used as standard anticancer drug. Total 61 lipophilic components were identified quantitatively from the sub-fraction of n-hexane in which saturated fatty acids (33.06%), unsaturated fatty acids (21.03%) and other organic components (43.35%). Twenty three hydrophilic components, 16 phenolic and 7 flavonoid compounds, were quantified in the hydrophilic sub-fractions obtained from the C. montanum roots. Among the hydrophilic components, the vanillic acid was analyzed as 46.463 ± 0.167 mg compound/g dry extract in the sub-fraction dichloromethane. The linoleic acid was analyzed as a substantial component (8.13%) in the sub-fraction n-hexane. The highest antiproliferative activities were obtained from the sub-fraction dichloromethane against HeLa cells (IC50 = 36.53 μg/mL) and the sub-fraction n-hexane against C6 cells (IC50 = 11.98 μg/mL). No cytotoxic effect was observed from these sub-fractions.




Similar content being viewed by others
References
Selvi S, Paksoy MY, Polat R, Cakilcioglu U (2014) Micromorphological and anatomical characteristics of the Genus Chrysophthalmum Schultz Bip. (Asteraceae) growing in Turkey. Proc Natl Acad Sci India B 84:431–438
Kirbag S, Zengin F, Kursat M (2009) Antimicrobial activities of extracts of some plants. Pak J Bot 41:2067–2070
Arasan S, Kaya I (2015) Some important plants belonging to asteraceae family used in folkloric medicine in Savur (Mardin/Turkey) area and their application areas. J Food Nutr Res 3:337–340
Siriwardhana N, Kalupahana NS, Moustaid-Moussa N (2012) Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 65:211–222
Bazinet RP, Laye S (2014) Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci 15:771–785
Demirtas I, Gecibesler IH, Yaglioglu AS (2013) Antiproliferative activities of isolated flavone glycosides and fatty acids from Stachys byzantina. Phytochem Lett 6:209–214
Yaglioglu AS, Demirtas I, Goren N (2014) Bioactivity-guided isolation of antiproliferative compounds from Centaurea carduiformis. Phytochem Lett 6:209–214
Adedapo AA, Adeoye BO, Sofidiya MO, Oyagbemi AA (2015) Antioxidant, antinociceptive and anti-inflammatory properties of the aqueous and ethanolic leaf extracts of Andrographis paniculata in some laboratory animals. J Basic Clin Physiol Pharmcol 26:327–334
Gupta R, Goyal R, Bhattacharya S, Dhar KL (2015) Antioxidative in vitro and antiosteoporotic activities of Prinsepia utilis Royle in female rats. Eur J Integr Med 7:157–163
Öksüz S, Topcu G, Krawiec M, Watson WH (1997) Eudesmanolides and other constituents of Inula thapsoides. Phytochemistry 46:1131–1134
Huo Y, Shi H, Wang M, Li X (2008) Complete assignments of 1H and 13C NMR spectral data for three sesquiterpenoids from Inula helenium. Magn Reson Chem 46:1208–1211
Cheng XR, Zhang SD, Wang CH, Ren J, Qin JJ, Tang X, Zhang WD (2013) Bioactive eudesmane and germacrane derivatives from Inula wissmanniana Hand.-Mazz. Phytochemistry 96:214–222
Topcu G, Öksüz S, Herz W, Diaz JG (1995) Structurally related guaianolides from Inula thapsoides. Phytochemistry 40:1717–1722
Konishi T, Shimada Y, Nagao T, Okabe H, Konoshima T (2002) Antiproliferative sesquiterpene lactones from the roots of Inula helenium. Biol Pharm Bull 25:1370–1372
Lokhande PD, Gawaı KR, Kodam KM, Kuchekar BS, Chabukswar AR, Jagdale SC (2007) Antibacterial activity of isolated constituents and extract of roots of Inula racemosa. Res J Med Plant 1:7–12
Gürbüz P, Dogan SD, Pasayeva L, Paksoy MY (2016) Guaiane-type sesquiterpene lactones from Chrysophthalmum montanum. Rec Natl Prod 10:714–720
Aytac Z, Anderberg AA (2001) A new species of Chrysophthalmum Schultz Bip. (Asteraceae-Inuleae) from Turkey. Bot J Linn Soc 137:211–214
Tanriover N, Ulu MO, Sanus GZ, Bilir A, Canbeyli R, Oz B, Akar Z, Kuday C (2008) The effects of systemic and intratumoral interleukin-12 treatment in C6 rat glioma model. Neurol Res 30:511–517
Ozcan G, Ozsoylemez OD, Akman G, Khalilia W, Yetiz BT, Karagoz A, Melikoglu G, Anil S, Kultur S, Sutlupinar N (2016) Screening for antitumor activity of various plant extracts on HeLa and C 4-I cell lines. JBUON 21:1552
Morales P, Maieves HA, Dias MI, Calhella RC, Sanchez-Mata MC, Santos-Buelga C, Ferreira IC (2017) Hovenia dulcis Thunb. pseudofruits as functional foods: phytochemicals and bioactive properties in different maturity stages. J Funct Food 29:37–45
Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17:299–305
Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, Yang CS (2009) Cancer preventive activities of tocopherols and tocotrienols. Carcinogenesis 205:1–62
Moreno MM, Olalla HM, Gimenez MR, Navarro AM, Rufian HJA (2015) Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J Food Compos Anal 38:19–26
Roleira FM, Tavares SEJ, Varela CL, Costa SC, Silva T, Garrido J, Borges F (2015) Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem 183:235–258
Erenler R, Sen O, Aksit H, Demirtas I, Yaglioglu AS, Elmastas M, Telci I (2016) Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. J Sci Food Agric 96:822–836
Gomes CA, Girao CT, Andrade JL, Milhazes N, Borges F, Marques MPM (2003) Anticancer activity of phenolic acids of natural or synthetic origin: a structure-activity study. J Med Chem 46:5395–5401
Chahar MK, Sharma N, Dobhal MP, Joshi YC (2011) Flavonoids: a versatile source of anticancer drugs. Pharmacogn Rev 5:1–12
Chan JSL, Asatiani MD, Sharvit LE, Trabelcy B, Barseghyan GS, Wasser SP (2015) Chemical composition and medicinal value of the new Ganoderma tsugae var. jannieae CBS-120304 medicinal higher basidiomycete mushroom. Int J Med Mushrooms 17:735–747
Kang HG, Jeong SH, Cho JH (2010) Antimutagenic and anticarcinogenic effect of methanol extracts of sweetpotato (Ipomea batata) leaves. Toxicol Res 26:29–35
Acknowledgements
The authors acknowledge the financial support of the Scientific Research Project, Department of Bingol University (Projects: BAP-21-217-2014 and BAP-21-322-2015). They also thank Dr. Alpaslan Kocak for the identification of plant material and Prof. Dr. Nazlı Arda and Associate Prof. Ali Karagöz for providing C6 and HeLa cell lines. They also thank Mr. Serkan Koldas for grammatical revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Significance statementChrysophthalmum montanum is used in the treatment of colds and sinuses. The apolar fractions rich in lipophilic components of C. montanum showed significant antiproliferative activities against HeLa and C6 cells as compared to 5-fluorouracil.
Rights and permissions
About this article
Cite this article
Gecibesler, I.H., Yaglıoglu, A.S., Gul, F. et al. Phytochemicals of Chrysophthalmum montanum (DC.) Boiss. Roots and Their Antiproliferative Activities Against HeLa and C6 Cell Lines. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 145–154 (2019). https://doi.org/10.1007/s40011-017-0925-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0925-1