Abstract
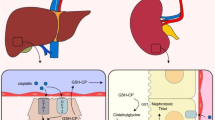
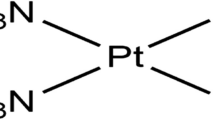
The present study was undertaken to investigate the cisplatin (cDDP) induced oxidative damage in hepatic tissue of wistar rats and mechanisms of protection by quercetin. A total of 24 wistar rats were randomly divided into four groups with six animals in each. Group I served as control, group II received cDDP (12 mg kg−1 body weight) and group III quercetin (50 mg kg−1 body weight) intra-peritoneally. Group IV received quercetin 6 h prior to cDDP administration intra-peritoneally. Administration of cDDP in rats resulted in significant (P < 0.05) elevation of plasma hepatic biomarkers, reduction of antioxidant system and marked histopathological alterations indicating acute hepatotoxicity. Treatment with quercetin prior to cDDP administration prevented hepatic dysfunctions as indicated by alterations in hepatic biomarkers, alleviated enzymatic and non-enzymatic components of the antioxidant system with reduced histopathological changes in hepatic tissue. The results suggest that cDDP induced hepatic damage is due to imbalance in oxidant and antioxidant system of hepatic tissue. The pretreatment with quercetin attenuated the oxidative damage induced by cDDP in hepatic tissue and this hepatoprotective effect of quercetin may be due to its direct scavenging of free radicals and/or enhancing antioxidant defense system of hepatic tissue in wistar rats.






Similar content being viewed by others
References
Arts IC, Hollman PC (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81(1 Suppl):317S–325S
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585(2–3):325–337
Okamoto T (2005) Safety of quercetin for clinical application (review). Int J Mol Med 16(2):275–278
Guo Y, Bruno RS (2015) Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem 26(3):201–210
De Boer VC, Dihal AA, Van der Woude H et al (2005) Tissue distribution of quercetin in rats and pigs. J Nutr 135(7):1718–1725
Arredondo F, Echeverry C, Abin-carriquiry JA et al (2010) After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 49(5):738–747
Liang L, Gao C, Luo M et al (2013) Dihydroquercetin (DHQ) induced HO-1 and NQO1 expression against oxidative stress through the Nrf2-dependent antioxidant pathway. J Agric Food Chem 61(11):2755–2761
Gan L, Johnson JA (2014) Oxidative damage and the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys Acta 1842(8):1208–1218
Kosaka T, Yamaguchi M, Motomura T, Mizuno K (2005) Investigation of the relationship between atherosclerosis and paraoxonase or homocysteine thiolactonase activity in patients with type 2 diabetes mellitus using a commercially available assay. Clin Chim Acta 359(1–2):156–162
Kilic SS, Aydin S, Kilic N, Erman F, Aydin S, Celik I (2005) Serum arylesterase and paraoxonase activity in patients with chronic hepatitis. World J Gastroenterol 11:7351–7354
Costa LG, Garrick JM, Roque PJ, Pellacani C (2016) Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016:2986796
Costa LG, Richter RJ, Li WF, Cole T, Guizzetti M, Furlong CE (2003) Paraoxonase (PON 1) as a biomarker of susceptibility for organophosphate toxicity. Biomarkers 8(1):1–12
Aviram M, Rosenblat M (2005) Paraoxonases and cardiovascular diseases: pharmacological and nutritional influences. Curr Opin Lipidol 16(4):393–399
Kiyici A, Okudan N, Gökbel H, Belviranli M (2010) The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J Med Food 13(3):725–728
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Francescato HD, Coimbra TM, Costa RS, Bianchi Mde L (2004) Protective effect of quercetin on the evolution of cisplatin-induced acute tubular necrosis. Kidney Blood Press Res 27(3):148–158
Behling EB, Sendão MC, Francescato HD, Antunes LM, Costa RS, Bianchi Mde L (2006) Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep 58(4):526–532
Verma PK, Raina R, Sultana M, Singh M, Kumar P (2016) Total antioxidant and oxidant status of plasma and renal tissue of cisplatin-induced nephrotoxic rats: protection by floral extracts of Calendula officinalis Linn. Renal Fail 38(1):142–150
Abf-Ellah MF, Aly HAA, Mokhlis HAM, Abdel-Aziz AH (2016) Quercetin attenuates di-(2-ethlyhexyl) phthalate-induced testicular toxicity in adult rats. Hum Exp Toxicol 35:232–243
Liao Y, Lu X, Lu C, Li G, Jin Y, Tang H (2008) Selection of agents for prevention of cisplatin-induced hepatotoxicity. Pharmacol Res 57(2):125–131
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 26:1231–1237
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Aycicek A, Erel O, Kocyigit A, Selek S, Demirkol MR (2006) Breast milk provides better antioxidant power than does formula. Nutrition 22:616–619
Motchnik AP, Frei B, Ames NB (1994) Measurement of antioxidants in human blood plasma, protein thiols. In: Packer L (ed) Oxygen radicals in biological systems, methods in enzymology, vol 234(D). California Academic Press, Millbrae, pp 273–274
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic, New York, pp 276–286
Marklund S, Marklund G (1974) Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 7:469–474
Hafeman DG, Sunde RA, Hoekstra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104:580–587
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Bio Chem 249(22):7130–7139
Koc A, Duru M, Ciralik H, Akcan R, Sogut S (2005) Protective agent, erdosteine, against cisplatin-induced hepatic oxidant injury in rats. Mol Cell Biochem 278:79–84
Naziroglu M, Karaoglu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195(2–3):221–230
Yilmaz HR, Sogut S, Ozyurt B, Ozugurlu F, Sahin S, Isik B et al (2005) The activities of liver adenosine deaminase, xanthine oxidase, catalase, superoxide dismutase enzymes and the levels of malondialdehyde and nitric oxide after cisplatin toxicity in rats: protective effect of caffeic acid phenethyl ester. Toxicol Ind Health 3–4:67–73
Sova P, Chladek J, Zak F et al (2005) Pharmacokinetics and tissue distribution of platinum in rats following single and multiple oral doses of LA-12 [(OC-6-43)-bis(acetato)(1-adamantylamine)amminedichloroplatinum(IV)]. Int J Pharm 288(1):123–129
Waseem M, Bhardwaj M, Tabassum H, Raisuddin S, Parvez S (2015) Cisplatin hepatotoxicity mediated by mitochondrial stress. Drug Chem Toxicol 38(4):452–459
Szentmihályi K, May Z, Szénási G et al (2014) Cisplatin administration influences on toxic and non-essential element metabolism in rats. J Trace Elem Med Biol 28(3):317–321
Jordan P, Carmo-Fonseca M (2000) Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci 57(8–9):1229–1235
Sallie R, Tredger JM, William R (1991) Drug and liver biopharmaceutical. Drug Disp 12:251–259
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4(10):1399–1440
Forbes S, Vig P, Poulsom R, Thomas H, Alison M (2002) Hepatic stem cells. J Pathol 197(4):510–518
Verma PK, Raina R, Sultana M, Prawez S, Jamwal N (2013) Hepatoprotective mechanisms of Ageratum conyzoides L. on oxidative damage induced by acetaminophen in wistar rats. Free Rad Antioxid 3:73–76
Iraz M, Ozerol E, Gulec M et al (2006) Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem Funct 24(4):357–361
Aviram M, Rosenblat M, Gaitini D et al (2004) Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23(3):423–433
Halliwell B, Gutteridge JM (1990) The antioxidants of human extracellular fluids. Arch Biochem Biophys 280(1):1–8
Myhrstad MC, Carlsen H, Nordström O, Blomhoff R, Moskaug JØ (2002) Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamyl cysteine synthetase catalytical subunit promoter. Free Radic Biol Med 32(5):386–393
Kim GN, Jang HD (2009) Protective mechanism of quercetin and rutin using glutathione metabolism on HO-induced oxidative stress in HepG2 cells. Ann N Y Acad Sci 1171:530–537
Kim GN, Kwon YI, Jang HD (2011) Protective mechanism of quercetin and rutin on 2,2′-azobis (2-amidinopropane) dihydrochloride or Cu2+-induced oxidative stress in HepG2 cells. Toxicol In Vitro 25(1):138–144
Omar HA, Mohamed WR, Arab HH, Arafa ESA (2016) Tangeretin alleviates cisplatin-induced acute hepatic injury in rats: targeting MAPKs and apoptosis. PLoS ONE 11(3):e0151649
Chirumbolo S (2010) The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets 9(4):263–285
Persons DL, Yazlovitskaya EM, Cui W, Pelling JC (1999) Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin Cancer Res 5:1007–1014
Acknowledgements
The authors thank to the Dean, Faculty of Veterinary Science and Animal Husbandry, R S Pura, Jammu for providing necessary facilities for conducting the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Significance statement
Cisplatin is an effective anticancer drug for the treatment of solid tumors with a limitation of potent nephrotoxicity and hepatotoxicity. To reduce its toxicity by using some natural compounds (quercetin) along with cisplatin can be an effective way for therapy. For this it is essential to know protective mechanisms for better utilization of natural compounds.
Rights and permissions
About this article
Cite this article
Verma, P.K., Raina, R., Prawez, S. et al. Protective Mechanisms of Quercetin on Cisplatin Induced Oxidative Damage in Hepatic Tissue of Wistar Rats. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 1399–1407 (2018). https://doi.org/10.1007/s40011-017-0877-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0877-5