Abstract
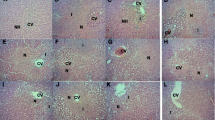
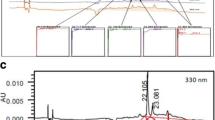
The present study elucidated the hepatoprotective activity of combination of alcohol water extracts (AWEs) of Murraya koenigii (AWEMK) and Phyllanthus niruri (AWEPN) leaves against paracetamol induced hepatic damage in ethanol exposed rats. Phytochemical analysis of AWEs determined the presence of flavonoids and phenols, followed by high performance liquid chromatography determination of an important antioxidant tannic acid in AWEMK and a major bioactive lignan phyllanthin in AWEPN. Gas chromatography mass spectrometric analysis revealed the presence of carbazole alkaloids in AWEMK whereas AWEPN was found rich in sterols. Prolonged consumption of ethanol has been reported to enhance the hepatic damage caused by paracetamol overdose by induction of cytochrome P450 (CYP450) and depletion of reduced glutathione (GSH) in alcohol abusers. Oral administration of alcohol (5 ml/kg/day) for three days followed by paracetamol (500 mg/kg, b.i.d.) for another 3 days produced liver damage in rats as manifested by the significant (P < 0.01) rise in levels of liver function tests and a marked increase in lipid peroxidation associated with significant reduction of the hepatic GSH and superoxide dismutase activity as compared with respective control values. Post-treatment of rats with silymarin (200 mg/kg), AWEMK (500 mg/kg), AWEPN (500 mg/kg) and combination of AWEMK and AWEPN (250 mg/kg each) for one week significantly (P < 0.01) inhibited these biochemical alterations which were confirmed by histopathological examination of the liver. Among all the treatment groups, the combination of extracts showed significant reversal of hepatic damage thus accentuating its hepatoprotective potential and immense value for use as a hepatoprotectant.




Similar content being viewed by others
References
Rastogi RP, Mehrotra BN (1998) Compendium of Indian medicinal plants (vol. 5). Central Drug Research Institute, Lucknow and National Institute of Science Communication, New Delhi, p 1059
Chowdhury JU, Bhuiyan MNI, Yusuf M (2008) Chemical composition of the essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J Pharma 3:59–63
Yusuf M, Chowdhury JU, Wahab MA, Begum J (1994) Medicinal Plants of Bangladesh. BCSIR Laboratories, Chittagong
Ningappa MB, Dinesha R, Srinivas L (2008) Antioxidant and free radical scavenging activities of polyphenol-enriched curry leaf (Murraya koenigii L.) extracts. Food Chem 106:720–728
Arulselvan P, Senthilkumar GP, Sathish KD, Subramanian S (2006) Anti-diabetic effect of Murraya koenigii leaves on streptozotocin induced diabetic rats. Pharmazie 61:874–877
Khuntia TK, Panda DS (2011) Evaluation of antibacterial, antifungal and anthelmintic activity of Murraya koenigii Spreng. Pharma Sci Monit 2(2):105–110
Muthumani P, Venkatraman S, Ramseshu KV, Meera R, Devi P, Kameswari B, Eswarapriya B (2009) Pharmacological studies of anticancer, anti inflammatory activities of Murraya koenigii (Linn) Spreng in experimental animals. J Pharm Sci Res 1(3):137–141
Noolu B, Ajumeera R, Chauhan A, Nagalla B, Manchala R, Ismail A (2013) Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complemt Altern Med 13:7–24
Sathaye S, Bagul Y, Gupta S, Kaur H, Redkar R (2011) Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp Toxicol Pathol 63:587–591
Paranjape P (2001) Indian medicinal plants: forgotten healers. Chaukhamba Sanskrit Pratisthan, Delhi, p 48
Makoshi MS, Adanyeguh IM, Nwatu ILI (2013) Hepatoprotective effect of Phyllanthus niruri aqueous extract in acetaminophen sub-acute exposure rabbits. J Vet Med Anim Health 5(1):8–15
Ahmed A, Hossain M, Amzad Ismail Z (2009) Antioxidant properties of the isolated flavonoids from the medicinal plant Phyllanthus niruri. Asian J Food Agro-Ind 2(03):373–381
Okoli CO, Obidike IC, Ezike AC, Akah PA, Salawu OA (2010) Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm Biol 49(3):248–255
Khanna AK, Rizvi F, Chander R (2002) Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol 82(1):19–22
Shin MS, Kang EH, Lee YI (2005) A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antiviral Res 67:163–168
Thomas SHL (1993) Paracetamol (acetaminophen) poisoning. Pharmacol Ther 60:91–120
Laine JE, Auriola S, Pasanen M, Juvonen RO (2009) Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 39(1):11–21
Das J, Ghosh J, Manna P, Sil PC (2010) Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Rad Res 44:340–355
Manyike PT, Kharasch ED, Kalhorn TF, Slattery JT (2000) Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin Pharmacol Ther 67:275–282
Zhao P, Slattery J (2002) Effects of ethanol dose and ethanol withdrawal on rat liver mitochondrial glutathione: implication of potentiated acetaminophen toxicity in alcoholics. Drug Metab Dispos 30:1413–1417
Zimmerman HJ, Maddrey WC (1995) Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 22:767–773
Kujala TS, Loponen JM, Klika KD, Pihlaja K (2000) Phenolics and betacyanins in red beetroot (Beta vulgaris) root: distribution and effect of cold storage on the content of total phenolics and three individual compounds. J Agric Food Chem 48:5338–5342
Kumar S, Kumar D, Saroha K, Singh N, Vashishta B (2008) Antioxidant and free radical scavenging potential of Citrullus Colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm 58:215–220
Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F (2005) Antioxidative activity of anthocyanins from purple sweet potato Ipomoera batatas cultivar Ayamurasaki. Biosci Biotechnol Biochem 69:979–988
Annamalai A, Lakshmi PTV (2009) HPTLC and HPLC analysis of bioactive phyllanthin from different organs of Phyllanthus amarus. Asian J Biotechnol 1(4):154–162
Pegg RB, Rybarczyk A, Amarowicz R (2008) Chromatographic separation of tannin fractions from a bearberry-leaf (Arctostaphylos uva-ursi L. Sprengel) extract by SE-HPLC-a short report. Pol J Food Nutr Sci 58:485–490
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Rehman SU (1984) Lead induced regional lipid peroxidation in brain. Toxicol Lett 21:333–337
Madesh M, Balasubramanian KA (1998) Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys 35(3):184–188
Woolson RF (1987) Statistical methods for the analysis of biomedical data. Wiley, New York
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Harish R, Shivanandappa T (2006) Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem 95:180–185
Chirdchupunseree H, Pramyothin P (2010) Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. J Ethnopharmacol 128:172–176
Nayak A, Mandal S, Banerji A, Banerji J (2010) Review on chemistry and pharmacology of Murraya koenigii Spreng (Rutaceae). J Chem Pharm Res 2(2):286–299
Khatun M, Billah M, Quader MA (2012) Sterols and sterol glucoside from Phyllanthus species. Dhaka Univ J Sci 60:5–10
Rajeshkumar NV, Joy KL, Girija K, Ramsewak RS, Nair MG, Ramadasan K (2002) Antitumour and anticarcinogenic activity of Phyllanthus amarus extract. J Ethnopharmacol 81:17–22
Mandal S, Nayak A, Kar M, Banerjee SK, Das A, Upadhyay SN, Singh RK, Banerji A, Banerji J (2009) Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia 81(1):72–74
Asghar SF, Rehman H, Choudahry MI, Rahman A (2011) Gas chromatography–mass spectrometry (GC–MS) analysis of petroleum ether extract (oil) and bio-assays of crude extract of Iris germanica. Int J Gen Mol Biol 3(7):95–100
Loizou S, Lekakis I, Chrousos GP, Moutsatsou P (2010) Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res 54(4):551–558
Sudha T, Chidambarampillai S, Mohan VR (2013) GC–MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd. J Appl Pharm Sci 3(05):126–130
Asif M, Kaushik S (2014) A mini review on solanesol: a trisesquiterpenoid alcohol of nicotiana tabacum. Int J Curr Pharm Sci 1(1):63–66
Rajeswari G, Murugan M, Mohan VR (2012) GC–MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res J Pharm Biol Chem Sci 3(4):302–308
Prachayasittikul S, Buraparuangsang P, Worachartcheewan A, Ayudhya CI, Ruchirawat S, Prachayasittikul V (2008) Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 13:904–921
Villalobos CA, Bull P, Sáez P, Cassels BK, Huidobro-Toro JP (2004) 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. Br J Pharmacol 141:1167–1174
Selvamangai C, Bhaskar A (2012) GCMS analysis of phytocomponents in the methanolic extract of Eupatorium triplinerve. Int J Drug Dev Res 4(4):148–153
Huang ZR, Lin YK, Fang JY (2009) Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 14:540–554
Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT (1993) Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem Pharm 45:1563–1569
Pradhan SC, Girish C (2006) Hepatoprotective herbal drug, silymarin- from experimental pharmacology to clinical medicine. Indian J Med Res 124:491–504
Herper HA (1961) The functions and tests of the liver. In: Review of physiological chemistry, Los Altos, California, Lange Medical Publishers pp 271–283
Reddy BS, Reddy RKK, Reddy BP, Ramakrishna S, Diwan PV (2008) Potential in vitro antioxidant and protective effects of Soymida febrifuga on ethanol induced oxidative damage in HepG2 cells. Food Chem Toxicol 46:3429–3442
Loganayaki N, Manian S (2010) In vitro antioxidant properties of indigenous underutilized fruits. Food Sci Biotechnol 19(3):725–734
Burk R (1983) Glutathione-dependent protection by rat liver microsomal protein against lipid per oxidation. Biochim Biophys Acta 757:21–28
Shen B, Chen H, Shen C, Xu P, Li J, Shen G, Yuan H, Han J (2015) Hepatoprotective effects of lignans extract from Herpetospermum caudigerum against CCl4-induced acute liver injury in mice. J Ethnopharmacol 164(22):46–52
Naidu VGM, Atmakur H, Katragadda SB, Devabakthuni B, Kota A, Reddy SCK, Kuncha M, Vishnu Vardhan MVPS, Kulkarni P, Janaswamy MR, Sistla R (2014) Antioxidant, hepatoprotective and cytotoxic effects of icetexanes isolated from stem-bark of Premna tomentosa. Phytomedicine 21(4):497–505
Yadav NP, Pal A, Shanker K, Bawankule DU, Gupta AK, Darokar MP, Khanuja SPS (2008) Synergistic effect of silymarin and standardized extract of Phyllanthus amarus against CCl4-induced hepatotoxicity in Rattus norvegicus. Phytomedicine 15:1053–1061
Mukazayire MJ, Minani V, Ruffo CK, Bizuru E, Stévigny C, Duez P (2011) Traditional phytotherapy remedies used in Southern Rwanda for the treatment of liver diseases. J Ethnopharmacol 138(2):415–431
Perera PK, Li Y (2012) Functional herbal food ingredients used in type 2 diabetes mellitus. Pharmacogn Rev 6(11):37–45
Acknowledgments
A special thanks is owed to Dr. A.H. Ahmed for providing his guidance and help during the whole study. Special gratitude is expressed towards Council of Scientific and Industrial Research for providing research fellowship for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shah, P., Singh, S.P., Gupta, A.K. et al. Combined Hepatoprotective Activity of Murraya koenigii and Phyllanthus niruri Extracts Against Paracetamol Induced Hepatotoxicity in Alcoholic Rats. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 655–665 (2018). https://doi.org/10.1007/s40011-016-0800-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0800-5