Abstract
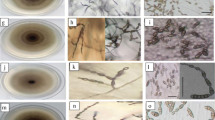
Early blight disease samples were collected from six different states of India. Seventeen different isolates of Alternaria spp were obtained in pure culture which were designated as PB-2, PB-4, PB-13, PB-17, PB-19, HR-12, HR-44, HR-62, MP-6, MP-7, JT-4, JT-11, UP-7, UP-10, UP-15, UP-20 and RJ-T-1. Characterization based on culture colour and conidial dimensions (conidial length and breadth, and beak length) indicated that no distinguished variation of the isolates was observed on the colour parameters. Although, a wide variation was observed in the length of conidia and beak, no significant difference was observed in conidial breadth except RJ-T-1. Among the isolates, conidial length of five isolates viz., HR-12, UP-7, UP-10, UP-15 and RJ-T-1 ranged from 80 to 97 µm whereas others were of significantly lesser size (30–51 µm). Similarly, significant difference was observed in beak length. The PCR amplification of the fungal DNA using universal primers ITS1 and ITS4 and sequencing indicated that, out of 17 isolates, 12 (JT-4, JT-11, HR-62, HR-44, PB-2, PB-4, PB-13, PB-17, PB-19, UP-20, MP-6 and MP-7) were closely matching with A. alternata (JX993756) and five isolates (HR-12, UP-7, UP-10, UP-15 and RJ-T-1) with A. solani (JF796068). Further, pathogenicity test on tomato revealed that both A. alternata and A. solani isolates were of virulent category indicating that former is also an incitant of early blight in northern India.
Similar content being viewed by others
References
Datar VV, Mayee CD (1981) Assessment of loss in tomato yield due to early blight. Indian Phytopathol 34:191–195
Gomes SMDTP, Carneiro EDB, Romano EP, Teixeira MZ, da Costa ME, Vasconcelos JCG (2010) Effect of biotherapic of Alternaria solani on the early blight of tomato-plant and the in vitro development of the fungus. Int J High Dilution Res 9:147–155
Derbalah AS, El-Mahrouk MS, El-Sayed AB (2011) Efficacy and safety of some plant extracts against tomato early blight disease caused by Alternaria solani. Plant Pathol J 10(3):115–121
Alhussan KM (2012) Morphological and physiological characterization of Alternaria solani isolated from tomato in Jordan Valley. Res J Biol Sci 7:316–319
Varma KP, Singh S, Gandhi SK (2007) Variability among Alternaria solani isolates causing early blight of tomato. Indian Phytopathol 60:180–186
Kumar V, Haldar S, Pandey KK, Singh RP, Singh AK, Singh PC (2008) Cultural, morphological, pathogenic and molecular variability amongst tomato isolates of Alternaria solani in india. World J Microbiol Biotechnol 24:1003–1009
Radhajeyalakshmi R, Velazhahan R, Samiyappan R, Doraiswamy S (2009) Systemic induction of pathogenesis related proteins (PRs) in Alternaria solani elicitor sensitized tomato cells as resistance response. Sci Res Essay 4:685–689
Bhatt JC, Gahlain A, Pant SK (2000) Record of Alternaria alternata on tomato, capsicum and spinach in Kumaon hills. Indian Phytopathol 53:495–496
Benlioglu S, Delen N (1996) Studies on the sporulation of the early blight agent of tomatoes. J Turk Phytopathol 25:23–28
Vunch RA, Rosner A, Stein A (1999) The use of the polymerase chain reaction (PCR) for detection of bean yellow mosaic virus in gladiolus. Ann Appl Biol 117:561–569
Bridge PD, Singh T, Arora DK (2004) The application of molecular markers in the epidemiology of plant pathogenic fungi. In: Arora DK, Bridge PD, Bhatnagar D (eds) Fungal biotechnology in agricultural, food, and environmental applications. Marcel Dekker Inc, New York, p 475
Abd-Esalem KA (2003) Non-gel based techniques for plant pathogen genotyping. Acta Microbiol Pol 52:329–341
Abd-Esalem KA, Asran-Amel A, Schneider F, Migheli Q, Verreet JA (2006) Molecular detection of Fusarium oxysporum. f. sp. vasinfectum in cotton roots by PCR and real-time PCR assay. J Plant Dis Prot 113:14–19
Bowmann KD, Albrecht U, Graham JH, Bright DB (2007) Detection of Phytophtora nicotianae and P. palmivora in citrus roots using PCR-RFLP in comparison with other methods. Eur J Plant Pathol 119:143–158
Manicom BQ, Bar-Joseph M, Rosner A, Vigodsky-Haas H, Kotze JM (1987) Potential applications of random DNA probes, and restriction fragment length polymorphisms in the taxonomy of Fusaria. Phytopathology 77:669–672
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a Guide to methods and applications. Academic Press Inc, New York, pp 315–322
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Pandey KK, Pandey PK, Kallo G, Banerjee MK (2003) Resistance to early blight of tomato with respect to various parameters of disease epidemics. J Gen Plant Pathol 69:364–371
Naik MK, Prasad Y, Bhat KV, Devika Rani GS (2010) Morphological, physiological, pathogenic and molecular variability among isolates of Alternaria solani from tomato. Indian Phytopathol 63:168–173
Kusaba M, Tsuge T (1995) Phylogeny of Alternaria fungi known to produce host-specific toxins on the basis of variation in internal transcribed spacers of ribosomal DNA. Curr Genet 28:491–498
Pryor BM, Gilbertson RL (2000) Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycol Res 104:1312–1321
Wang HK, Zhang TY, Zhang M (2001) Application of sequencing of 5.8S rDNA, ITS1 and ITS2 on identification and classification of Alternaria at species level. Mycosystema 20:168–173
Pryor BM, Michailides TJ (2002) Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 92:406–416
Guo LD, Xu L, Zheng WH, Hyde KD (2004) Genetic variation of Alternaria alternata, an endophytic fungus isolated from Pinus tabulaeformis as determined by random amplified microsatellites (RAMS). Fungal Divers 16:53–65
Acknowledgments
The present work was carried-out with the financial support of ICAR-Out Reach Project on Diagnosis and Management of Leaf Spot Diseases of Field and Horticultural Crops under EFC of ICAR - Indian Institute of Horticultural Research, Bangalore, India. There is no conflict of interest regarding the research work done.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loganathan, M., Venkataravanappa, V., Saha, S. et al. Morphological, Pathogenic and Molecular Characterizations of Alternaria Species Causing Early Blight of Tomato in Northern India. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 86, 325–330 (2016). https://doi.org/10.1007/s40011-014-0446-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-014-0446-0