Abstract
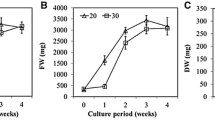
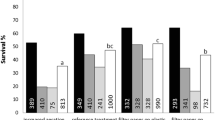
Embryogenic suspensions of Pinus kesiya (Khasi pine) derived from embryogenic cultures obtained from immature zygotic embryos (intact megagametophytes) of Pk-04 genotype were grown in shake-flasks and self-designed bubble bioreactors. Conifer somatic embryogenesis could find its utility for rapid mass propagation if grown as suspension cultures. To minimize the destruction of embryogenic cultures during study, a few growth characteristics viz., sedimented cell volume and number of stage-I (early stage) and -III (cotyledonary) embryo formation in suspensions were determined over a 21-day period. Compared to shake-flasks, proliferation in bioreactors resulted in increased biomass. The suspension culture showed a mixture of freely suspended as well as aggregates of stage-I (early stage) embryos. Maturation of somatic embryos was done on gelled medium and in submerged culture where gelled solid medium was covered with a layer of liquid medium. The number of stage-III (cotyledonary) embryos formed in bubble bioreactor were significantly higher than shake-flask cultures with both solid medium and submerged cultures. The cotyledonary embryos germinated successfully producing plantlets on germination medium.




Similar content being viewed by others
References
Preil W (1991) Application of bioreactors in plant propagation. In: Deburg PC, Zimmer RH (eds) Micropropagation, technology and application. Kluwer Academic Publishers, Dordrecht, pp 425–445
Takayama S, Akita M (1994) The types of bioreactors used for shoots and embryos. Plant Cell Tissue Organ Cult 39:147–156
Andrea-Kodym F, Zapata-Arias J (2001) Low cost alternatives for the micropropagation of banana. Plant Cell Tissue Organ Cult 66:67–71
Mehrotra S, Goel MK, Kukreja AK, Mishra BN (2007) Efficiency of liquid culture systems over conventional micropropagation: a progress towards commercialization. Afr J Biotechnol 6(13):1484–1492
Rathore JS, Rai MK, Phulwaria M, Shekhwat NS (2013) A liquid culture system for improved micropropagation of mature Acacia nilotica (L.) Del. Spp. indica and ex vitro rooting. Proc Natl Acad Sci India B. doi:10.1007/s4001101302048
Hvoslef-Eide AK, Olsen OAS, Lyngved R, Munster C, Heyerdahl PH (2005) Bioreactor design for propagation of somatic embryos. Plant Cell Tissue Organ Cult 81:265–276
Ibaraki Y, Kurata K (2001) Automation in somatic embryo production. Plant Cell Tissue Organ Cult 65:179–199
Takayama S, Akita M (2005) Practical aspects of bioreactor application in mass propagation of plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 61–78
Ziv M (2005) Simple bioreactors for mass propagation of plants. Plant Cell Tissue Organ Cult 81:277–285
Furusaki S, Takeda T (2011) Bioreactors for plant cell culture. In: Moo-Young M (ed) Comprehensive biotechnology, vol 2. Elsevier, Amsterdam, pp 361–372
Ebil R, Brandli J, Ebil D (2012) Plant cell bioreactors. In: Doelle HW, Rokem S, Berovic M (eds) Biotechnology, encyclopaedia of life support systems (EOLSS). EOLSS Publishers, Oxford (www.eolss.net)
Georgiev MI, Ebil R, Zhong J–J (2013) Hosting the plant cells in vitro: recent trends in bioreactors. Appl Microbiol Biotechnol 97:3787–3800. doi:10.1007/s00253-013-4817-x
Kendall HW, Beachy R, Eisner T, Gould F, Herdt R, Raven PH, Schell JS, Swaminathan MS (1997) Bioengineering of crops: report of the World Bank. Environ Soc Sustain Dev Monogr Ser No 23, World Bank, Washington, DC
Powledge TM (1984) Biotechnology touches the forest. Biotechnology 2:763–772
James C, Krattiger AF (1996) Global review of the field testing and commercialisation of transgenic plants: 1986 to 1995, the first decade of crop biotechnology. International services for the acquisition of agri-biotech applications (ISAAA) Brief No 1, ISAAA, Ithaca, NY
Bishop-Hurley SL, Zabkiewicz L, Grace L (2001) Conifer genetic engineering: transgenics Pinus radiata (D. Don) and Picea abies (Karst) plants are resistant to herbicide Buster. Plant Cell Rep 20:235–243
Tautorus TE, Lulsdorf MM, Kikcio SI, Dunstan DI (1994) Nutrient utilisation during bioreactor culture, and regeneration of somatic embryo cultures of Picea mariana and Picea glauca-engelmannii. In Vitro Cell Dev Biol Plant 30:58–63
Attree SM, Pomeroy MK, Fowke LC (1994) Production of vigorous, desiccation tolerant white spruce (Picea glauca [Moench.] Voss.) synthetic seeds in a bioreactor. Plant Cell Rep 13:601–606
Kvaalen H (1997) Some preliminary results with embryogenic cell suspensions of Norway spruce cultured in bioreactors. COST 822 Working Group 2, 5–8 Sept 1996, Sanremo, Italy. COST 822 Development of integrated systems for large-scale propagation of elite plants using in vitro techniques. Report of activities 1996. European Commission 1997, pp 338
Krogstrup P (1990) Effect of culture densities on cell proliferation and regeneration from embryogenic cell suspensions of Picea sitchensis. Plant Sci 72:115–123
Choudhury H, Kumaria S, Tandon P (2008) Induction and maturation of somatic embryos from intact megagametophyte explants in Khasi pine (Pinus kesiya Royle ex. Gord.). Curr Sci 95(10):1433–1438
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
Ingram B, Mavituna F (2000) Effect of bioreactor configuration on the growth and maturation of Picea sitchensis somatic embryo cultures. Plant Cell Tissue Organ Cult 61:87–96
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Lulsdorf M, Tautorus TE, Kikcio SI, Dunstan DI (1992) Growth parameters of embryogenic spruce (Picea glauca-engelmannii complex) and black spruce (Picea mariana Mill.). Plant Sci 82:227–234
Patnaik J, Sahoo S, Debata BK (1997) Somatic embryogenesis and plantlet regeneration from cell suspension cultures of palmarosa grass (Cymbopogon martini). Plant Cell Rep 16:430–434
Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 95–116
Scragg AH (2007) The production of flavours by plant cell cultures. In: Berger RG (ed) Flavours and fragrances: chemistry, bioprocessing and sustainability. Springer, Berlin, pp 599–614
Merchuk JC, Gluz M (1999) Bioreactors, air-lift reactors. In: Ficklinger MC, Stephen WD (eds) Encyclopaedia of bioprocess technology: fermentation, biocatalysis, bioseparation, vol 1. Wiley, New York, pp 320–353
Acknowledgments
Laboratory facilities provided by the Department of Botany and other logistics by the Department of Basic Sciences and Social Sciences, North-Eastern Hill University, Shillong, Meghalaya, India are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choudhury, H., Tandon, P. Non-Destructive Assessment of Growth Performance of Embryogenic Suspension Culture of Pinus kesiya (Royle ex. Gord.) in Shake-Flask and Self-Designed Bubble Bioreactor and Successful Regeneration of Plantlets from the Culture Systems. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 84, 771–777 (2014). https://doi.org/10.1007/s40011-013-0253-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-013-0253-z