Abstract
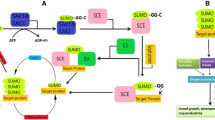
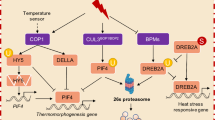
Heat stress is an important factor affecting the agricultural production. At the molecular level post translational modifications have been shown to be influenced in plants under heat stress. The authors have studied palmitoylation, sumoylation, S-nitrosylation and nitration in the plant gene sequences associated or not associated with heat stress. In the present paper it has been shown that sumoylation is an important property which is possibly influences a large number of proteins associated with heat stress in a wide variety of plants. This property can be utilized for identifying novel sequences associated with heat stress in plants.
Similar content being viewed by others
References
Wahid A, Gelani S, Ashraf M, Foolad (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61(199):223
Ashraf M, Harrris PJC (eds) (2010) Abiotic stresses plant resistance through breeding and molecular approaches, 2nd edn. CRC Press Taylor & Francis Group, Haworth, New York
Ohn TB, Anderson P (2010) The role of posttranslational modifications in the assembly of stress granules. Wiley Interdiscip Rev RNA 1:486–493
Seo J, Lee KJ (2004) Post-translational modifications and their biological functions: proteomic analysis and systematic approaches. J Biochem Mol Biol 37:35–44
Shi J, Kim KN, Ritz O, Albrecht V, Gupta R, Harter K, Luan S, Kudla J (1999) Novel protein kinases associated with calcineurin B-like calcium sensors in Arabidopsis. Plant Cell 11:2393–2405
Cheong YH, Kim KN, Pandey GK, Gupta R, Grant JJ, Luan S (2003) CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 15:1833–1845
Albrecht V, Weinl S, Blazevic D, D’Angelo C, Batistic O, Kolukisaoqlu U, Bock R, Schulz B, Harter K, Kudla J (2003) The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J 36:457–470
Batistic O, Sorek N, Schultke S, Yalovsky S, Kudla J (2008) Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell 20:1346–1362
Ludwig AA, Romeis T, Jones JDG (2004) CDPK-mediated signalling pathways: specificity and cross-talk. J Exp Bot 55:181–188
Martin ML, Busconi L (2000) Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J 24:429–435
Leclercq J, Ranty B, Sanchez-Ballesta MT, Li Z, Jones B, Jauneau A, Pech JC, Latche A, Ranjeva R, Bouzayen M (2005) Molecular and biochemical characterization of LeCRK1, a ripening-associated tomato CDPK-related kinase. J Exp Bot 56:25–35
Conti L, Price G, O’Donnell E, Schwessinger B, Dominy P, Sadanandom A (2008) Small ubiquitin-like modifier proteases overly tolerant to salt1 and -2 regulate salt stress response in Arabidopsis. Plant Cell 20:2894–2908
Kurepa J, Walker JM, Smalle J, Gosink MM, Davis SJ, Durham TL, Sung DY, Vierstra RD (2003) The small ubiquitin-like modifier(SUMO) protein modification system in Arabidopsis. Accumulation of SUMO1 and -2 conjugates is increased by stress. J Biol Chem 278:6862–6872
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-Nitrosylated protein in Arabidopsis. Plant Physiol 137:921–930
Corpas FJ, del Rio LA, Barroso JB (2007) Need of biomarkers of nitrosative stress in plants. Trends Plant Sci 12:436–438
Cecconi D, Orzetti S, Vandelle E, Rinalducci S, Zolla L, Delledonne M (2009) Protein nitration during defense response in Arabidopsis Thaliana. Electrophoresis 30:2460–2468
Xue Y, Liu Z, Cao J, Ren J (2011) Computational prediction of post-translational modification sites in proteins. In: Systems and computational biology-molecular and cellular experimental systems. InTech. doi: 10.5772/18559, ISBN 978-953-307-280-7
Liu H, Wong (2003) Data mining tools for biological sequences. J Bioinf Comput Biol 01:139–167
Hatzidamianos G, Diplaris S, Athanasiadis I, Mitkas PA (2003) GenMiner: a data mining tool for protein analysis. In: 9th Panhellenic conference in informatics, Thessaloniki, Greece, November 21–23. pp 346–360
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A., Vats, G., Chandra, N. et al. Sumoylation May Play an Important Role in Modification of Large Number of Proteins Associated with Heat Stress in Plants. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 84, 709–712 (2014). https://doi.org/10.1007/s40011-013-0249-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-013-0249-8