Abstract
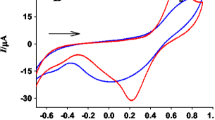
A metal sensor based on the incorporation of fibrous tissues of coconut shell in carbon paste matrix is presented for the determination of lead ions in aqueous solution. Cyclic voltammetry and electrochemical impedance study were used to characterize the electrochemical parameters of the coconut shell modified carbon paste electrode. Lead ions were pre-concentrated on the modified electrode surface at open circuit potential and the determination was carried out by stripping voltammetry in anodic direction. Various factors affecting the sensitivity and precision of the determination of lead, including accumulating solvent, pH of accumulating solvent, accumulation time and stripping solvent were optimized. The effect of the surface active macro molecules was also studied using Triton X-100, cetyl trimethyl ammonium bromide and sodium dodecyl sulfate as representative for neutral, cationic and anionic surfactants. Interference with other metals on the determination of lead was studied. Under optimum experimental conditions i.e., acetate buffer of pH 5 as an accumulating solvent, 0.1 M HCl as stripping solvent and 15 min accumulation time linear calibration curve was obtained in the concentration range 120–400 μg l−1 with limit of detection 80μg l−1.







Similar content being viewed by others
References
Liu HJ, Qu L, Hu S, Zhan TR, Zhao CZ, Sun W (2010) Sensitive and simple electrochemical detection of lead(II) with carbon ionic liquid electrode. J Chin Chem Soc 57:1367–1373
Senkal BF, Ince M, Yavuz E, Yaman M (2007) The synthesis of new polymeric sorbent and its application in preconcentration of cadmium and lead in water samples. Talanta 72(3):962–967
John AC, Ibironke LO, Adedeji, Victor, Oladunni O (2011) Equilibrium and kinetic studies of the biosorption of heavy metal (cadmium) on cassia siamea bark. Am Euras J Sci Res 6(3):123–130
Guidelines for drinking-water quality (2006) Recommendations (1). WHO Library Cataloguing-in-Publication Data
Eletta OAA (2007) Determination of some trace metal levels in Asa river using AAS and XRF techniques. Int J Phys Sci 2(3):056–060
Lee SK, Choi HS (2001) Spectrophotometric determination of cadmium and copper with ammonium pyrrolidine dithiocarbamate in nonionic tween 80 micellar media. Bull Korean Chem Soc 22(5):463–466
Mokgalaka NS, McCrindle RI, Botha BM (2004) Multielement analysis of tea leaves by inductively coupled plasma optical emission spectrometry using slurry nebulisation. J Anal At Spectrom 19:1375–1378
Brainina KZ, Malakhova NA, Stojko NY (2000) Stripping voltammetry in environmental and food analysis. Fresen J Anal Chem 368:307–325
Economou A (2005) Bismuth-film electrodes: recent developments and potentialities for electroanalysis. Trends Anal Chem 24:334–340
Manivannan A, Kawasaki R, Tryk DA, Fujishima A (2004) Interaction of Pb and Cd during anodic stripping voltammetric analysis at boron-doped diamond electrodes. Electrochim Acta 49(20):3313–3318
Ganjali MR, Aghabalazadeh S, Khoobi M, Ramazani A, Foroumadi A, Shafiee A, Norouzi P (2011) Nanocomposite based carbon paste electrode for selective analysis of copper. Int J Electrochem Sci 6:52–62
Sar E, Berber H, Asc B, Cankurtaran H (2008) Determination of some heavy metal ions with a carbon paste electrode modified by poly(glycidyl methacrylate-methyl methacrylate-divinylbenzene) microspheres functionalized by 2-aminothiazole. Electroanal 20(14):1533–1541
Adraoui I, Rhazi M, Amine A, Idrissi L, Curulli A, Palleschic G (2005) Lead determination by anodic stripping voltammetry using a p-phenylenediamine modified carbon paste electrode. Electroanal 17(8):685–693
Lu M, Toghill KE, Compton RG (2011) Simultaneous detection of trace cadmium(II) and Lead(II) using an unmodified edge plane pyrolytic graphite electrode. Electroanal 23(5):1089–1094
Tonle IK, Letaief S, Ngameni E, Walcarius A, Detellier C (2011) Square wave voltammetric determination of lead(II) ions using a carbon paste electrode modified by a thiol-functionalized kaolinite. Electroanal 23(1):245–252
Goubert-Renaudin S, Moreau M, Despas C, Meyer M, Denat F, Lebeau B, Walcariusa A (2009) Voltammetric detection of lead(II) using amide-cyclam-functionalized silica-modified carbon paste electrodes. Electroanal 21(15):1731–1742
Svancara I, Baldrianova L, Tesarova E, Hocevar SB, Elsuccary SAA, Economou A, Sotiropoulos S, Ogorevc B, Vytras K (2006) Recent advances in anodic stripping voltammetry with bismuth-modified carbon paste electrodes. Eletroanal 18(2):177–185
Sun D, Wan C, Li G, Wu K (2007) Electrochemical determination of lead using a montmorillonite calcium-modified carbon paste electrode. Microchim Acta 158(3–4):255–260
Hassan RYA, Habib IHI, Hassan HNA (2008) Voltammetric determination of lead(II) in medical lotion and biological samples using chitosan-carbon paste electrode. Int J Electrochem Sci 3:935–945
Mojica ERE, Vidal JM, Pelegrina AB, Micor JRL (2007) Voltammetric determination of Pb(II) ions at carbon paste electrode modified with banana tissue. J Appl Sci 7(9):1286–1292
Rajawat DS, Satsangee SP (2011) Voltammetric determination of Pb(II) ions by carbon paste electrode modified with lemon grass powder. Res J Chem Environ 15(3):55–60
Esmeraldo MA, Barreto ACH, Freitas JEB, Fachine PBA, Sombra ASB, Corradini E, Mele G, Maffezzoli A, Mazzetto SE (2010) Dwarf green coconut fibers: a versatile natural renewable raw bioresource. Treatment, morphology and physicochemical properties. Bioresources 5(4):2478–2501
Gupta A, Yadav R, Devi P (2011) Removal of hexavalent chromium using activated coconut shell and activated coconut coir as low cost adsorbent. Spec issue front ind microbiol environ biotechnol 2(3):8–12
Sousa FW, Oliveira AG, Ribeiro JP, Rosa MF, Keukeleire D, Nascimento RF (2010) Green coconut shells applied as adsorbent for removal of toxic metal ions using fixed-bed column technology. J Environ Manag 91:1634–1640
Pino GH, Mesquita LMS, Torem ML, Pinto GAS (2006) Biosorption of cadmium by green coconut shell powder. Miner Eng 19:380–387
Rajawat DS, Kardam A, Srivastava S, Satsangee SP (2012) Nano cellulosic fibers modified carbon paste electrode for ultra trace determination of Cd(II) and Pb(II) in aqueous solution. Environ Sci Pollut Res. doi:10.1007/s11356-012-1194-4
Rajawat DS, Srivastav S, Satsangee SP (2012) Electrochemical determination of mercury at trace levels using Eichhornia crassipes modified carbon paste electrode. Int J Electrochem Sci 7:11456–11469
Rajawat DS, Kardam A, Srivastava S, Satsangee SP (2013) Adsorptive stripping voltammetric technique for monitoring of mercury ions in aqueous solution using nano cellulosic fibers modified carbon paste electrode. Nat Aca Sci Lett. doi:10.1007/s40009-013-0116-4
Bartlett PN, Denuarret G, Souza MFB (2000) A study of the preconcentration and stripping voltammetry of Pb(II) at carbon electrodes. Analyst 125:1135–1138
Mojica ERE, Florinia EM, Micor JRL (2002) Fiber of kapok (Ceiba pentandra) as component of a metal sensor for lead in water samples. Philipine J Crop Sci 27(2):37–42
Acknowledgments
The authors gratefully acknowledge Prof. V. G. Dass, Director, Dayalbagh Educational Institute, Dayalbagh, Agra, for providing necessary research facilities. The authors also thank Ministry of Human Resource and Development, New Delhi for rendering financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajawat, D.S., Srivastava, S. & Satsangee, S.P. Mercury Free Anodic Stripping Voltammetric Determination of Pb(II) Using a Low Cost “Cocos nucifera” Shell Modified Carbon Paste Electrodes. Natl. Acad. Sci. Lett. 37, 547–553 (2014). https://doi.org/10.1007/s40009-014-0276-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40009-014-0276-x