Abstract
Purpose
Superdisintegrants are typically used at low concentrations in tablets. As a result, the spatial distribution of disintegrant particles within the tablet may be inhomogeneous, resulting in varied disintegration times. This study aimed to investigate the effect of disintegrant spatial distribution on tablet disintegratability.
Methods
Tablets with various degrees of disintegrant spatial distribution were engineered using a novel experimental design. The effects of relative spatial distribution of disintegrant particles on tablet tensile strength, liquid penetration rate and disintegration time were investigated.
Results
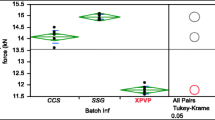
It was observed that increased clustering of disintegrant particles generally promoted faster tablet disintegration due to more localized swelling and strain recovery of sodium starch glycolate and crospovidone, respectively. However, for tablets with insoluble fillers and sodium starch glycolate, a high degree of disintegrant clustering prolonged disintegration due to the formation of gel plugs which impeded liquid penetration into the tablet and caused the tablet to break up into floccules instead. Tablets made with insoluble fillers were also found to be more sensitive to changes in disintegrant spatial distribution compared to those containing soluble fillers.
Conclusion
Overall, the effects of disintegrant spatial distribution were dependent on the type of disintegrant and filler used.






Similar content being viewed by others
References
Al-Sharabi M, Markl D, Mudley T, Bawuah P, Karttunen AP et al (2020) Simultaneous investigation of the Liquid Transport and Swelling Performance during Tablet Disintegration. Int J Pharm 584:119380
Alderborn G (2013) Tablets and compaction. In: Michael E, Aulton (eds) Pharmaceutics: the Science of Dosage Form Design, 4th edn. Churchill Livingstone, Edinburgh/New York, pp 397–440
Augsburger LL, Brzeczko AW, Shah U, Hahm HA (2007) Super disintegrants: characterization and function. In: James Swarbrick (ed) Encyclopedia of Pharmaceutical Technology, 3rd edn. Informa Healthcare, New York, pp 3553–3567
Basaleh S, Bisharat L, Cespi M, Berardi A (2020) Temperature: an overlooked factor in Tablet Disintegration. Eur J Pharm Sci 151:105388
Bauhuber S, Warnke G, Berardi A (2021) Disintegrant selection in Hydrophobic Tablet formulations. J Pharm Sci 110:2028–2037
Berardi A, Bisharat L, Blaibleh A, Pavoni L, Cespi M (2018) A simple and inexpensive image analysis technique to Study the Effect of disintegrants Concentration and diluents type on disintegration. J Pharm Sci 107:2643–2652
Bolhuis GK, Armstrong NA (2006) Excipients for direct Compaction-An update. Pharm Dev Technol 11:111–124
Curlin LC (1955) A note on Tablet Disintegration with Starch. J Am Pharm Assoc 44:16
Desai PM, Liew CV, Heng PWS (2012) Understanding disintegrant action by visualization. J Pharm Sci 101:2155–2164
Desai PM, Er PX, Liew CV, Heng PWS (2014) Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by Design Approach. AAPS PharmSciTech 15:1093–1104
Edge S, Steele DF, Staniforth JN, Chen A, Woodcock PM (2002) Powder Compaction Properties of Sodium Starch Glycolate disintegrants. Drug Dev Ind Pharm 28:989–999
Ekmekciyan N, Tuglu T, El-Saleh F, Muehlenfeld C, Stoyanov E et al (2018) Competing for water: a New Approach to Understand Disintegrant performance. Int J Pharm 548:491–499
Faroongsarng D, Peck GE (1994) The Swelling & Water Uptake of tablets III: moisture sorption behavior of Tablet disintegrants. Drug Dev Ind Pharm 20:779–798
Gissinger D, Stamm A (1980) A comparative evaluation of the properties of some Tablet disintegrants. Drug Dev Ind Pharm 6:511–536
Haware RV, Chaudhari PD, Parakh SR, Bauer-Brandl A (2008) Development of a Melting Tablet containing promethazine HCl against Motion Sickness. AAPS PharmSciTech 9:1006–1015
Hiew TN, Johan NB, Desai PM, Chua SM, Loh ZH et al (2016) Effect of moisture sorption on the performance of Crospovidone. Int J Pharm 514:322–331
Johnson JR, Wang LH, Gordon MS, Chowhan ZT (1991) Effect of Formulation Solubility and Hygroscopicity on Disintegrant Efficiency in tablets prepared by Wet Granulation, in terms of dissolution. J Pharm Sci 80:469–471
Khan KA, Rhodes CT (1975) Water-Sorption properties of Tablet Disintegrants. J Pharm Sci 64:447–451
Kissa E (1996) Wetting and Wicking. Text Res J 66:660–668
Kondo A, Koide T, Fukami T (2023) Evaluation of the effect of disintegrant distribution on the dissolution behavior of Pharmaceutical tablets using Raman Chemical Imaging. Chem Pharm Bull (Tokyo) 71:454–458
Lendlein A, Kelch S (2002) Shape-memory polymers. Angew Chem Int Ed 41:2034–2057
Lewis EN, Carroll JE, Clarke F (2001) A Near Infrared View of Pharmaceutical Formulation Analysis. NIR News 12:16–18
Maclean N, Walsh E, Soundaranathan M, Khadra I, Mann J et al (2021) Exploring the performance-controlling Tablet Disintegration mechanisms for Direct Compression formulations. Int J Pharm 599:120221
Markl D, Maclean N, Mann J, Williams H, Abbott A et al (2021) Tablet Disintegration performance: Effect of Compression pressure and storage conditions on Surface Liquid absorption and swelling kinetics. Int J Pharm 601:120382
Moreton RC (2008) Disintegrants in Tableting. In: Larry L, Augsburger, Hoag SW (eds) Pharmaceutical Dosage forms: tablets. CRC Press, New York, pp 217–249
Nogami H, Hasegawa J, Miyamoto M (1967) Studies on Powdered preparations. XX. Disintegration of the aspirin tablets containing starches as Disintegrating Agent. Chem Pharm Bull (Tokyo) 15:279–289
Nogami H, Nagai T, Fukuoka E, Sonobe T (1969) Disintegration of the aspirin tablets containing Potato Starch and Microcrystalline Cellulose in various concentrations. Chem Pharm Bull (Tokyo) 17:1450–1455
Patel NR, Hopponent RE (1966) Mechanism of action of Starch as a disintegrating Agent in Aspirin tablets. J Pharm Sci 55:1065–1068
Patel S, Kaushal AM, Bansal AK (2007) Effect of particle size and Compression Force on Compaction Behavior and Derived Mathematical parameters of Compressibility. Pharm Res 24:111–124
Quodbach J, Kleinebudde P (2016) A critical review on Tablet Disintegration. Pharm Dev Technol 21:763–774
Quodbach J, Moussavi A, Tammer R, Frahm J, Kleinebudde P (2014) Tablet Disintegration studied by high-resolution real-time magnetic resonance imaging. J Pharm Sci 103:249–255
Rigby SP, Van Der Walle CF, Raistrick JH (2004) Determining drug spatial distribution within controlled delivery tablets using MFX Imaging. J Control Release 96:97–100
Rojas J, Guisao S, Ruge V (2012) Functional Assessment of four types of disintegrants and their effect on the Spironolactone Release Properties. AAPS PharmSciTech 13:1054–1062
Rowe RC, Sheskey PJ, Quinn ME, American Pharmacists A (2009) Handbook of Pharmaceutical excipients. Pharmaceutical Press, London
Rudnic EM, Rhodes CT, Welch S, Bernardo P (1982) Evaluations of the mechanism of Disintegrant Action. Drug Dev Ind Pharm 8:87–109
Sacchetti M, Teerakapibal R, Kim K, Elder EJ Jr (2017) Role of Water Sorption in Tablet Crushing Strength, Disintegration, and dissolution. AAPS PharmSciTech 18:2214–2226
Shotton E, Leonard GS (1972) The Effect of Intra- and Extragranular Maize Starch on the disintegration of compressed tablets. J Pharm Pharmacol 24:798–803
Skelbæk-Pedersen AL, Al-Sharabi M, Vilhelmsen TK, Rantanen J, Zeitler JA (2020) Effect of particle size and deformation behaviour on Water Ingress into tablets. Int J Pharm 587:119645
So C, Narang AS, Mao C (2021) Modeling the tablet disintegration process using the Finite Difference Method. J Pharm Sci 110:3614–3622
Soundaranathan M, Al-Sharabi M, Sweijen T, Bawuah P, Zeitler JA et al (2023) Modelling the evolution of Pore structure during the disintegration of Pharmaceutical tablets. Pharmaceutics 15:489
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B (2014) Microcrystalline Cellulose, a Direct Compression Binder in a quality by Design Environment-A Review. Int J Pharm 473:64–72
Turkoglu M, Sakr A (2009) Tablet Dosage forms. In: Alexander T, Florence, Juergen, Siepmann (eds) Modern pharmaceutics, 5th edn. Informa Healthcare USA, New York, pp 481–497
Twitchell AM (2013) Mixing. In: Michael E, Aulton (eds) Pharmaceutics: the Science of Dosage Form Design, 4th edn. Churchill Livingstone, Edinburgh/New York, pp 170–186
Young PM, Edge S, Staniforth JN, Steele DF, Price R (2005) Dynamic Vapor Sorption properties of Sodium Starch Glycolate disintegrants. Pharm Dev Technol 10:249–259
Zhao N, Augsburger LL (2005) The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of Hydrochlorothiazide from directly compressed tablets. AAPS PharmSciTech 6:E120–E126
Zhao J, Koo O, Pan D, Wu Y, Morkhade D et al (2017) The impact of disintegrant type, surfactant, and API properties on the processability and performance of Roller compacted formulations of Acetaminophen and Aspirin. AAPS J 19:1387–1395
Zheng AY, Heng PWS, Chan LW (2022) Tablet Disintegratability: sensitivity of superdisintegrants to temperature and Compaction pressure. Pharmaceutics 14:2725
Acknowledgements
The authors acknowledge the financial support provided by GEA-NUS PPRL fund (N-148-000-008-001). Audrey Yi Zheng is a recipient of the National University of Singapore Graduate Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (A.Y. Zheng, W.W. Huang, L.Y.J. Poon, E.S. Wong, P.W.S. Heng and L.W. Chan) declare that they have no conflict of interest.
Research involving human or animal participants
This article does not contain any studies with human and animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, A.Y., Huang, W.W., Poon, L.Y.J. et al. Examining the effect of spatial distribution of disintegrant particles on tablet disintegratability. J. Pharm. Investig. 54, 195–207 (2024). https://doi.org/10.1007/s40005-023-00639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00639-6