Abstract
Purpose
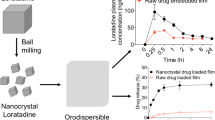
Loratadine (LOR), a commonly prescribed antihistamine, has low water solubility but high permeability. In this study, an orodispersible film incorporating the nanoparticulate loratadine was prepared to enhance the oral bioavailability of a poorly water-soluble drug.
Methods
Nanoparticulate loratadine was formulated using the antisolvent precipitation method and optimized by a single-factor design based on the particle size and polydispersity index. The optimal formulation was spray-dried and characterized by powder X-ray diffraction and differential scanning calorimetry. Nanoparticulate loratadine was loaded into an orodispersible film using a solvent casting method.
Results
In the dissolution tests, the nanoparticulate loratadine-loaded orodispersible film exhibited a 6.5-fold higher dissolution rate than the pure loratadine-loaded film and a similar dissolution rate compared to the commercialized orodispersible tablet, Loratadine SPM. In pharmacokinetic studies conducted on rats, the maximum concentration (Cmax) and area under the curve of the plasma concentration–time profile from 0 to 24 h (AUC0-24 h) of the nanoparticulate loratadine-loaded orodispersible film significantly increased 1.8-fold and 5.8-fold, respectively. The elimination half-life (t1//2) increased 5.1-fold compared to the loratadine-loaded counterpart.
Conclusion
These results suggest the potential of orodispersible films to improve the oral bioavailability of poorly water-soluble drugs and promote compliance in pediatric and geriatric patients.






Similar content being viewed by others
References
Albayati TM, Salih IK, Alazzawi HF (2019) Synthesis and characterization of a modified surface of SBA-15 mesoporous silica for a chloramphenicol drug delivery system. Heliyon 5:e02539
Alshweiat A, Katona G, Csóka I, Ambrus R (2018) Design and characterization of loratadine nanosuspension prepared by ultrasonic-assisted precipitation. Eur J Pharm Sci 122:94–104
Boateng JS, Stevens HNE, Eccleston GM et al (2009) Development and mechanical characterization of solvent-cast polymeric films as potential drug delivery systems to mucosal surfaces. Drug Dev Ind Pharm 35:986–996
Comoglu T, Dilek Ozyilmaz E (2019) Orally disintegrating tablets and orally disintegrating mini tablets—novel dosage forms for pediatric use. Pharm Dev Technol 24:902–914
D’Addio SM, Prud’homme RK (2011) Controlling drug nanoparticle formation by rapid precipitation. Adv Drug Deliv Rev 63:417–426
Dalvi SV, Dave RN (2009) Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind Eng Chem Res 48:7581–7593
Danaei M, Dehghankhold M, Ataei S et al (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57
Deng J, Huang L, Liu F (2010) Understanding the structure and stability of paclitaxel nanocrystals. Int J Pharm 390:242–249
Desai P, Wang KZ, Ann D et al (2020) Efficacy and pharmacokinetic considerations of loratadine nanoformulations and its combinations for pancreatic cancer chemoprevention. Pharm Res 37:21
Diaz DA, Colgan ST, Langer CS et al (2016) Dissolution similarity requirements: how similar or dissimilar are the global regulatory expectations? AAPS J 18:15–22
Frizon F, de Oliveira EJ, Donaduzzi CM et al (2013) Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol 235:532–539
Hoffmann EM, Breitenbach A, Breitkreutz J (2011) Advances in orodispersible films for drug delivery. Expert Opin Drug Deliv 8:299–316
Kapote DN, Wagner KG (2021) Influence of shellac on the improvement of solubility and supersaturation of loratadine amorphous solid dispersion using a new grade of HPMC. J Drug Deliv Sci Technol 61:102116
Khan MZI, Raušl D, Zanoški R et al (2004) Classification of loratadine based on the biopharmaceutics drug classification concept and possible in Vitro–in Vivo Correlation. Biol Pharm Bull 27:1630–1635
Madhav KV, Kishan V (2018) Self microemulsifying particles of loratadine for improved oral bioavailability: preparation, characterization and in vivo evaluation. J Pharm Investig 48:497–508
Melzig S, Finke JH, Schilde C, Kwade A (2018) Formation of long-term stable amorphous ibuprofen nanoparticles via antisolvent melt precipitation (AMP). Eur J Pharm Biopharm 131:224–231
Nguyen KV, Dang TK, Pham HT et al (2022) Development of Panax notoginseng saponins-loaded orodispersible films: a potential approach to enhance delivery efficacy in older adults. J Appl Pharm Sci 12:44–53
Raju PN, Kumar MS, Reddy CM, Ravishankar K (2013) Formulation and evaluation of fast dissolving films of loratidine by solvent casting method. Pharma Innov 2:5
Roman IJ, Danzig MR (1993) Loratadine. a review of recent findings in pharmacology, pharmacokinetics, efficacy, and safety, with a look at its use in combination with pseudoephedrine. Clin Rev Allergy 11:89–110
Sarheed O, Shouqair D, Ramesh K et al (2020) Physicochemical characteristics and in vitro permeation of loratadine solid lipid nanoparticles for transdermal delivery. Ther Deliv 11:685–700
Sinha B, Müller RH, Möschwitzer JP (2013) Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int J Pharm 453:126–141
Sora DI, Udrescu S, David V, Medvedovici A (2007) Validated ion pair liquid chromatography/fluorescence detection method for assessing the variability of the loratadine metabolism occurring in bioequivalence studies. Biomed Chromatogr 21:1023–1029
Sun DD, Wen H, Taylor LS (2016) Non-sink dissolution conditions for predicting product quality and in vivo performance of supersaturating drug delivery systems. J Pharm Sci 105:2477–2488
Thorat AA, Dalvi SV (2012) Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: recent developments and future perspective. Chem Eng J 181–182:1–34
Tran TH, Poudel BK, Marasini N et al (2013) Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. Int J Pharm 443:50–57
Tran TB, Tran TH, Vu YH et al (2021) pH-responsive nanocarriers for combined chemotherapies: a new approach with old materials. Cellulose 28:3423–3433
Truong DH, Tran TH, Ramasamy T et al (2015) Preparation and characterization of solid dispersion using a novel amphiphilic copolymer to enhance dissolution and oral bioavailability of sorafenib. Powder Technol 283:260–265
Truong DH, Tran TH, Ramasamy T et al (2016) Development of solid self-emulsifying formulation for improving the oral bioavailability of erlotinib. AAPS PharmSciTech 17:466–473
Üner M, Karaman EF, Aydoğmuş Z (2014) Solid Lipid nanoparticles and nanostructured lipid carriers of loratadine for topical application: physicochemical stability and drug penetration through rat skin. Trop J Pharm Res 13:653–660
Van Eerdenbrugh B, Van den Mooter G, Augustijns P (2008) Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm 364:64–75
Van Eerdenbrugh B, Vermant J, Martens JA et al (2009) A screening study of surface stabilization during the production of drug nanocrystals. J Pharm Sci 98:2091–2103
Vasoya JM, Desai HH, Gumaste SG et al (2019) Development of solid dispersion by hot melt extrusion using mixtures of polyoxylglycerides with polymers as carriers for increasing dissolution rate of a poorly soluble drug model. J Pharm Sci 108:888–896
Vikash, Kumar V (2020) Ultrasonic-assisted de-agglomeration and power draw characterization of silica nanoparticles. Ultrason Sonochem 65:105061
Wang J, Chang R, Zhao Y et al (2017) Coamorphous loratadine-citric acid system with enhanced physical stability and bioavailability. AAPS PharmSciTech 18:2541–2550
Wu L, Zhang J, Watanabe W (2011) Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev 63:456–469
Xia D, Wu JX, Cui F et al (2012) Solvent-mediated amorphous-to-crystalline transformation of nitrendipine in amorphous particle suspensions containing polymers. Eur J Pharm Sci 46:446–454
Yin OQP, Shi X, Chow MSS (2003) Reliable and specific high-performance liquid chromatographic method for simultaneous determination of loratadine and its metabolite in human plasma. J Chromatogr B 796:165–172
Zhang H-X, Wang J-X, Zhang Z-B et al (2009) Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm 374:106–113
Zhu W-Z, Wang J-X, Shao L et al (2010) Liquid antisolvent preparation of amorphous cefuroxime axetil nanoparticles in a tube-in-tube microchannel reactor. Int J Pharm 395:260–265
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (Khanh Van Nguyen, Thu Kim Dang, Linh Thi Dieu Vu, Nhan Thi Ha, Hieu Duy Truong, and Tuan Hiep Tran) declare that they have no conflict of interest.
Statement of human and animal rights
Animal studies were performed after receiving approval from the Animal Ethics Experimentation Committee of Vietnam Military Medical University No. LORA.01 (Hanoi, Vietnam).
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The data that support the findings of this study are available from the corresponding author, on special request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Nguyen, K., Dang, T.K., Vu, L.T.D. et al. Orodispersible film incorporating nanoparticulate loratadine for an enhanced oral bioavailability. J. Pharm. Investig. 53, 417–426 (2023). https://doi.org/10.1007/s40005-023-00613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-023-00613-2