Abstract
Purpose
The purpose of this study was to develop a patient-centric formulation of tegoprazan, a novel potassium-competitive acid blocker, using modified-release drug-coated pellets.
Methods
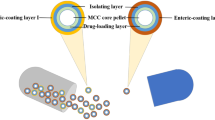
Tegoprazan tablets, sustained-release (SR) polymer-coated pellets, SR drug-coated pellets, enteric-coated (DR) pellets, SR polymer-coated pellets with an enteric coating (CR1), SR drug-coated pellets with an enteric coating (CR2), and tegoprazan immediate-release (IR) powder were prepared. Dual-release capsules were prepared by combining the IR powder with the CR1, CR2, and DR pellets (IR/CR1, IR/CR2, and IR/DR, respectively). In vitro dissolution tests were conducted to screen the formulations, and in vivo pharmacokinetic (PK) analysis was conducted using cynomolgus monkeys. Design of experiments was employed to optimize the formulation for the CR2 pellets. The effect of the particle size on the dissolution profile of the CR2 pellets was investigated using scanning electron microscopy.
Results
In vitro dissolution of the IR/CR1 capsule produced the desired pH- and time-dependent release profile, and in vivo PK analysis confirmed that drug absorption was well-controlled by this formulation. The IR/CR2 capsule exhibited a pH- and time-dependent release of tegoprazan that was similar to that of the IR/CR1 capsule. Small drug particles appeared to become embedded in the densely packed coating, thus dissolving more slowly than the medium and large drug particles.
Conclusion
Unlike the CR1 pellets, the CR2 pellets did not require an additional coating and curing process. The developed IR/CR2 capsule is also expected to prevent nocturnal acid breakthrough and thus improve patient compliance.






Similar content being viewed by others
References
Ammar HO, Ghorab MM, Felton LA, Gad S, Fouly AA (2015) Effect of antiadherents on the physical and drug release properties of acrylic polymeric films. AAPS PharmSciTech 17:682–692
Bodmeier R, Paeratakul O (1994) The effect of curing on drug release and morphological properties of ethylcellulose pseudolatex-coated beads. Drug Dev Ind Pharm 20(9):1517–1533
Boparai V, Rajagopalan J, Triadafilopoulos G (2008) Guide to the use of proton pump inhibitors in adult patients. Drugs 68:925–947
Brown G (1956) Formation of films from polymer dispersions. J Polym Sci 22(102):423–434
Capece M, Barrows J, Davé RN (2015) Controlled release from drug microparticles via solventless dry-polymer coating. J Pharm Sci 104(4):1340–1351
Carlin B, Li J, Felton L (2008) Chapter 1. Pseudolatex dispersions for controlled drug delivery. In: Felton LA, McGinity JW (eds) Aqueous polymeric coatings for pharmaceutical dosage forms, 3rd edn. Informa Healthcare, London, pp 1–46
De Jaeghere W, De Beer T, Van Bocxlaer J, Remon JP, Vervaet C (2015) Hot-melt extrusion of polyvinyl alcohol for oral immediate release applications. Int J Pharm 492(1–2):1–9
Dey NS, Majumdar S, Rao MEB (2008) Review article − Multiparticulate drug delivery systems for controlled release. Trop J Pharm Res 7(3):1067–1075
Dürig T, Fassihi R (1997) Mechanistic evaluation of binary effects of magnesium stearate and talc as dissolution retardants at 85% drug loading in an experimental extended-release formulation. J Pharm Sci 86(10):1092–1098
Dutta U, Moayyedi P (2013) Management of reflux-related symptoms. Best Pract Res Clin Gastroenterol 27:387–400
Fass R, Chey WD, Zakko SF, Andhivarothai N, Palmer RN et al (2009) Clinical trial: the effects of the proton pump inhibitor dexlansoprazole MR on daytime and nighttime heartburn in patients with non-erosive reflux disease. Aliment Pharmacol Ther 29(12):1261–1272
Fass R, Johnson DA, Orr WC, Han C, Mody R et al (2011) The effect of dexlansoprazole MR on nocturnal heartburn and GERD-related sleep disturbances in patients with symptomatic GERD. Am J Gastroenterol 106(3):421–431
Fass R, Inadomi J, Han C, Mody R, O’Neil J, Perez MC (2012) Maintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified release. Clin Gastroenterol Hepatol 10(3):247–253
Horn J (2006) Review article: understanding the pharmacodynamic and pharmacokinetic differences between proton pump inhibitors—focus on pKa and metabolism. Aliment Pharmacol Ther 2:340–350
Józó M, Simon N, Yi L, Móczó J, Pukánszky B (2022) Improved release of a drug with poor water solubility by using electrospun water-soluble polymers as carriers. Pharmaceutics 14(1):34
Kevra A (2005) Evaluation of a film coating that produces enhanced tablet-to-tablet film uniformity. AAPS J 7(S2):1811
Kim B, Byun Y, Lee EH (2021) DoE-Based design of a simple but efficient preparation method for a non-rffervescent gastro-retentive floating tablet containing metformin HCl. Pharmaceutics 13(8):1225
Laine L, Katz PO, Johnson DA, Ibegbu L, Goldstein MJ et al (2011) Randomised clinical trial: a novel rabeprazole extended release 50 mg formulation vs. esomeprazole 40 mg in healing of moderate-to-severe erosive oesophagitis. The results of two double-blind studies. Aliment Pharmacol Ther 33:203–212
Matsukawa J, Hori Y, Nishida H, Kajino M, Inatomiet N (2011) A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 81:1145–1151
Mehta R, Teckoe J, Schoener C, Workentine S, Ferrizzi D et al (2016) Investigation into the effect of ethylcellulose viscosity variation on the drug release of metoprolol tartrate and acetaminophen extended release multiparticulates—Part I. AAPS PharmSciTech 17:1366–1375
Mendizabal E, Castellanos-Ortega JR, Puig JE (1992) A method for selecting a polyvinyl alcohol as stabilizer in suspension polymerization. Colloids Surf 63:209–217
Meruva S, Thool P, Shah S, Karki S, Bowen W (2019) Formulation and performance of irbesartan nanocrystalline suspension and granulated or bead-layered dried powders – Part I. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2019.03.007
Morelli G, Chen H, Rossiter G, Rege B, Lu Y (2011) An open-label, parallel, multiple-dose study comparing the pharmacokinetics and gastric acid suppression of rabeprazole extended-release with esomeprazole 40 mg and rabeprazole delayed-release 20 mg in healthy volunteers. Aliment Pharmacol Ther 33:845–854
Mori H, Suzuki H (2019) Role of acid suppression in acid-related diseases: proton pump inhibitor and potassium-competitive acid blocker. J Neurogastroenterol Motil 25:6–14
Muschert S, Siepmann F, Leclercq B, Siepmann J (2011) Dynamic and static curing of ethylcellulose:PVA–PEG graft copolymer film coatings. Eur J Pharm Biopharm 78:455–461
Prasad BS, Chinna EM, Kamalakar RG (2018) Effect of polysorbate 80 and particle size of budesonide API on in-vitro dissolution profiles of budesonide MUPS tablets 9 mg. Res J Pharm Technol 11(10):4285–4295
Qazi F, Shoaib MH, Yousuf RI, Siddiqui F, Nasiri MI et al (2020) QbD based eudragit coated meclizine HCl immediate and extended release multiparticulates: formulation, characterization and pharmacokinetic evaluation using HPLC-Fluorescence detection method. Sci Rep. https://doi.org/10.1038/s41598-020-71751-y
Rajabi-Siahboomi A, Farrell TP (2008) The applications of formulated systems for the Aqueous film coating of pharmaceutical oral solid dosage forms. In: Felton LA, McGinity JW (eds) Aqueous polymeric coatings for pharmaceutical dosage forms, 3rd edn. Informa Healthcare, London, pp 323–343
Salehi H, Karimi M, Raofie F (2021) Micronization and coating of bioflavonoids extracted from Citrus sinensis L. peels to preparation of sustained release pellets using supercritical technique. J Iran Chem Soc 18:3235–3248
Salib NN, El-menshaway ME, Ismail AA (1976) Ethyl cellulose as a potential sustained release coating for oral pharmaceuticals. Pharmazie 31(10):721–723
Shah VP, Tsong Y, Sathe P, Liu JP (1998) In vitro dissolution profile comparison—statistics and analysis of the similarity factor, f2. Pharm Res 15(6):889–896
Shin JM, Sachs G (2002) Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology 123:1588–1597
Sodeman WA, Sodeman TC (2005) Proton pump inhibitors: patient and caregiver's guide. In: Sodeman WA, Sodeman TC (ed) Instructions for geriatric patients, 3rd ed. Elsevier Saunders, pp 133−134.
Story MJ (1977) Enteric-coated pellets: theoretical analysis of effect of dispersion in the stomach on blood level profiles. J Pharm Sci 66(10):1495–1496
Strand DS, Kim D, Peura DA (2017) 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 11(1):27–37
Takahashi N, Take Y (2018) Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther 364:275–286
Vakily M, Zhang W, Wu J, Atkinson SN, Mulford D (2009) Pharmacokinetics and pharmacodynamics of a known active PPI with a novel dual delayed release technology, dexlansoprazole MR: a combined analysis of randomized controlled clinical trials. Curr Med Res Opin 25:627–638
Wang Y, Yang M, Shen R, Shao S, Chen L et al (2018) Development of metoprolol tartrate-loaded sustained-release pellets: effect of talc on the mechanism of drug release. Pharm Dev Technol 23(7):664–673
Yang E, Kim S, Kim B, Kim B, Kim Y et al (2022) Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.15268
Yoon DY, Sunwoo J, Shin N, Kim AR, Kim B et al (2021) Effect of meal timing on pharmacokinetics and pharmacodynamics of tegoprazan in healthy male volunteers. Clin Transl Sci 14(3):934–941
Acknowledgements
This research was supported by HK inno.N Corp. and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF- 2020M3E5D9080795). The authors thank all researchers involved in the tegoprazan program at HK inno.N Corp.
Conflict of interest
All authors (S.C. Lee, M. Kim, D. Kim, E.K. Jeon, and E.H. Lee) declare that they have no conflicts of interest.
Statement of human and animal rights
All experimental procedures regarding the evaluation of pharmacokinetic characteristics in non-clinical models were performed following the regulations of the Animal Experimental Ethics Committee (IACUC, Institutional Animal Care and Use Committee, Approval No. ORIENT-IACUC-20124).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, S.C., Kim, M., Kim, D. et al. Development of a patient-centric formulation of tegoprazan, a novel potassium-competitive acid blocker, using modified-release drug-coated pellets. J. Pharm. Investig. 52, 623–638 (2022). https://doi.org/10.1007/s40005-022-00582-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-022-00582-y