Abstract
Propose
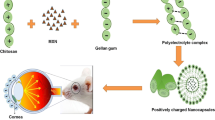
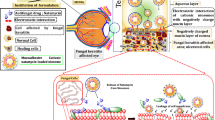
Fungal keratitis is one of the major causes in loss of vision and blindness. Amphotericin B (AmB) is the first-line treatment of keratitis caused by Candida spp. and related fungi. Due to its high molecular weight and low aqueous solubility, it hampers the ocular bioavailability. It is well-known that γ-cyclodextrin (γCD) can enhance AmB solubility. The aim of this study was to investigate the effect of the additives on γCD solubilization of AmB and develop AmB eye drop nanosuspensions.
Methods
0.18% (w/v) AmB nanosuspensions containing either 0.25% (w/v) chitosan or 0.10% (w/v) phospholipids (PL) in aqueous γCD eye drop medium were prepared by using high pressure homogenization technique. Physicochemical properties, in vitro mucoadhesive, in vitro permeation, degree of AmB aggregation, hemolytic activity and in vitro antifungal test were determined.
Results
The physicochemical properties of the nanosuspensions containing AmB/γCD nanoaggregates were in the acceptable range. The particle size of all formulations was in nanometer range. The degree of aggregation was less than 2.0 that meant the drug was not in the aggregate form when solubilized in γCD. These formulations demonstrated relatively low hemolytic activity and effectively improved the antifungal activity.
Conclusion
AmB/γCD nanosuspension stabilized by 0.10% (w/v) PL is the promising formulation for further studies due to that it was excellent physical appearance, had mucoadhesive property, enhanced in vitro permeation, had low toxicity and improved the antifungal activity.





Similar content being viewed by others
References
Akshay K, Himanshu B, Ashish P, Sandip B, Shailesh S, Ketan R (2014) Development of albendazole nanosuspension by various techniques. Drug Deliv Lett 4(2):87–95
Ansari Z, Miller D, Galor A (2013) Current thoughts in fungal keratitis: diagnosis and treatment. Curr Fungal Infect Rep 7(3):209–218
Banker GS, Rhodes CT (2002) Modern pharmaceutics, 4th edn. Marcel Dekker, New York
Barwicz J, Christian S, Gruda I (1992) Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob Agents Chemother 36(10):2310–2315
Bin Choy Y, Park J-H, Prausnitz MR (2008) Mucoadhesive microparticles engineered for ophthalmic drug delivery. J Phys Chem Solids 69(5–6):1533–1536
Carrier RL, Miller LA, Ahmed I (2007) The utility of cyclodextrins for enhancing oral bioavailability. J Control Release 123(2):78–99
Charvalos E, Tzatzarakis MN, Van Bambeke F, Tulkens PM, Tsatsakis AM, Tzanakakis GN et al (2005) Water-soluble amphotericin B–polyvinylpyrrolidone complexes with maintained antifungal activity against Candida spp. and Aspergillus spp. and reduced haemolytic and cytotoxic effects. J Antimicrob Chemother 57(2):236–244
Chhonker YS, Prasad YD, Chandasana H, Vishvkarma A, Mitra K, Shukla PK et al (2015) Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol 72:1451–1458
Clinical and Laboratory Standards Institute (2008a) Reference method for broth dilution antifungal susceptibility testing of yeast; Approved Standard-Third Edition. CLSI document M27-A3, Wayne, PA.
Clinical and Laboratory Standards Institute (2008b) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard-Second Edition. CLSI document M38-A2, Wayne, PA.
Constantin M, Fundueanu G, Bortolotti F, Cortesi R, Ascenzi P, Menegatti E (2004) Preparation and characterisation of poly(vinyl alcohol)/cyclodextrin microspheres as matrix for inclusion and separation of drugs. Int J Pharm 285(1):87–96
Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP, Torrado JJ (2008) Effect of aggregation state on the toxicity of different amphotericin B preparations. Int J Pharm 361(1):64–69
Fanos V, Cataldi L (2000) Amphotericin B-induced nephrotoxicity: a review. J Chemother 12(6):463–470
Funasaki N, Ishikawa S, Neya S (2002) Binding of short-chain lecithin by β-cyclodextrin. Langmuir 18(5):1786–1790
Gaitano GG, Brown W, Tardajos G (1997) Inclusion complexes between cyclodextrins and triblock copolymers in aqueous solution: a dynamic and static light-scattering study. J Phy Chem B 101(5):710–719
Hamilton-Miller JMT (1973) The effect of pH and of temperature on the stability and bioactivity of nystatin and amphotericin B. J Pharm Pharmacol 25(5):401–407
Hargreaves PL, Nguyen T-S, Ryan RO (2006) Spectroscopic studies of amphotericin B solubilized in nanoscale bilayer membranes. Biochim Biophys Acta 1758(1):38–44
Ing LY, Zin NM, Sarwar A, Katas H (2012) Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int J Biomater 2012:632698
Jansook P, Pichayakorn W, Muankaew C, Loftsson T (2016) Cyclodextrin–poloxamer aggregates as nanocarriers in eye drop formulations: dexamethasone and amphotericin B. Drug Dev Ind Pharm 42(9):1446–1454
Jansook P, Pichayakorn W, Ritthidej GC (2018) Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): effect of drug loading and biopharmaceutical characterizations. Drug Dev Ind Pharm 44(10):1693–1700
Jansook P, Stefánsson E, Thorsteinsdóttir M, Sigurdsson BB, Kristjánsdóttir SS, Bas JF et al (2010) Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm 76(2):208–214
Javed I, Hussain SZ, Ullah I, Khan I, Ateeq M, Shahnaz G et al (2015) Synthesis, characterization and evaluation of lecithin-based nanocarriers for the enhanced pharmacological and oral pharmacokinetic profile of amphotericin B. J Mater Chem B 3(42):8359–8365
Kagan S, Ickowicz DE, Domb AJ, Dagan A, Polacheck I (2016) Unique aggregation of conjugated amphotericin B and its interaction with lipid membranes. Med Mycol 55(4):414–421
Kajtár M, Vikmon M, Morlin E, Szejtli J (1989) Aggregation of amphotericin B in the presence of γ-cyclodextin. Biopolymers 28(9):1585–1596
Kayser O, Olbrich C, Yardley V, Kiderlen AF, Croft SL (2003) Formulation of amphotericin B as nanosuspension for oral administration. Int J Pharm 254(1):73–75
Kim J-C, Lee E-O, Kim J-Y, Bae SK, Choi T-B, Kim J-D (1997) Hemolytic and antifungal activity of liposome-entrapped amphotericin b prepared by the precipitation method. Pharm Dev Tech 2(3):275–284
Kouchak M, Bahmandar R, Bavarsad N, Farrahi F (2016) Ocular dorzolamide nanoliposomes for prolonged iop reduction: in-vitro and in-vivo evaluation in rabbits. Iran J Pharm Res 15(1):205–212
Krauland AH, Alonso MJ (2007) Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int J Pharm 340(1):134–142
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453(1):167–180
Loftssona T, Järvinen T (1999) Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev 36(1):59–79
Ludwig A (2005) The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 57(11):1595–1639
Miranda JCd, Martins TEA, Veiga F, Ferraz HG (2011) Cyclodextrins and ternary complexes: technology to improve solubility of poorly soluble drugs. Braz J Pharm Sci 47:665–681
Nogueiras-Nieto L, Sobarzo-Sánchez E, Gómez-Amoza JL, Otero-Espinar FJ (2012) Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: implications in drug solubilization and delivery. Eur J Pharm Biopharm 80(3):585–595
Rajagopalan N, Chen SC, Chow W-S (1986) A study of the inclusion complex of amphotericin-B with γ-cyclodextrin. Int J Pharm 29(2):161–168
Rasheed A, Kumar AKC, Sravanthi VVNSS (2008) Cyclodextrins as drug carrier molecule: a review. Sci Pharm 76(4):567–598
Rautaraya B, Sharma S, Kar S, Das S, Sahu SK (2011) Diagnosis and treatment outcome of mycotic keratitis at a tertiary eye care center in eastern India. BMC Ophthalmol 11:39
Rochelle do Vale Morais A, Silva AL, Cojean S, Balaraman K, Bories C, Pomel S et al (2018) In-vitro and in-vivo antileishmanial activity of inexpensive Amphotericin B formulations: Heated Amphotericin B and Amphotericin B-loaded microemulsion. Exp Parasitol 192:85–92
Rodriguez-Perez AI, Rodriguez-Tenreiro C, Alvarez-Lorenzo C, Concheiro A, Torres-Labandeira JJ (2006) Drug solubilization and delivery from cyclodextrin-pluronic aggregates. J Nanosci Nanotechnol 6(9–10):3179–3186
Ruiz HK, Serrano DR, Dea-Ayuela MA, Bilbao-Ramos PE, Bolás-Fernández F, Torrado JJ et al (2014) New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int J Pharm 473(1):148–157
Tananuvat N, Salakthuantee K, Vanittanakom N, Pongpom M, Ausayakhun S (2012) Prospective comparison between conventional microbial work-up vs PCR in the diagnosis of fungal keratitis. Eye (Lond) 26(10):1337–1343
Tanito M, Hara K, Takai Y, Matsuoka Y, Nishimura N, Jansook P et al (2011) Topical dexamethasone-cyclodextrin microparticle eye drops for diabetic macular edema. Invest Ophthalmol Vis Sci 52(11):7944–7948
Urtti A, Salminen L (1993) Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol 37(6):435–456
Wiest DB, Maish WA, Garner SS, El-Chaar GM (1991) Stability of Amphotericin B in four concentrations of dextrose injection. Am J Hosp Pharm 48(11):2430–2433
Yu BG, Okano T, Kataoka K, Kwon G (1998) Polymeric micelles for drug delivery: solubilization and haemolytic activity of amphotericin B. J Control Release 53(1):131–136
Zhiwen Y, Min L, Jian C, Weijun F, Yanli Z, Man Y et al (2014) Development and characterization of amphotericin b nanosuspensions for oral administration through a simple top-down method. Curr Pharm Biotechnol 15(6):569–576
Zhou Y, Fang Q, Niu B, Wu B, Zhao Y, Quan G et al (2018) Comparative studies on amphotericin B nanosuspensions prepared by a high pressure homogenization method and an antisolvent precipitation method. Colloids Surf B 172:372–379
Zia Q, Khan AA, Swaleha Z, Owais M (2015) Self-assembled amphotericin B-loaded polyglutamic acid nanoparticles: preparation, characterization and in vitro potential against Candida albicans. Int J Nanomedicine 10:1769–1790
Acknowledgements
This research is kindly supported by the Ratchadapisek Sompot Fund, Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jansook, P., Maw, P.D., Soe, H.M.S.H. et al. Development of amphotericin B nanosuspensions for fungal keratitis therapy: effect of self-assembled γ-cyclodextrin. J. Pharm. Investig. 50, 513–525 (2020). https://doi.org/10.1007/s40005-020-00474-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-020-00474-z