Abstract
Purpose
One of the primary challenges in developing highly concentrated protein formulations is ensuring adequate stability of the protein product. Among the many available methods, cold solvent precipitation is useful, and it has been adopted for protein purification. This study aimed to characterize protein stability under various precipitation conditions and to determine the most stable precipitation conditions.
Methods
Lysozyme was used as a model protein to investigate the effects of various solvents and temperature conditions on the physicochemical properties. Size exclusion chromatography, dynamic light scattering, circular dichroism, and thermodynamic analysis are the primary tools for the protein characterization.
Results
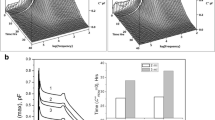
Changes in the rehydrated particle size, secondary structure, thermodynamic stability, and in vitro activity of lysozyme were observed under various conditions. By analyzing the effect of temperature on protein characteristics, it has been discovered that protein precipitation at a temperature of 4 °C or below is optimal. During the precipitation at – 20 °C, the secondary structures of proteins are adversely affected by the formation of ice crystals. None of the solvents used as precipitants in this study affected the activity of the protein. However, changes in the secondary structure were minimal when ethanol and octanol were used.
Conclusion
In this study, the feasibility of various precipitation conditions was evaluated for stable reversible precipitation formation. Although further studies are needed, precipitation of proteins using various organic solvents could be used in the development of formulations that purify and concentrate proteins.






Similar content being viewed by others
References
Alves M, Vieira NS, Rebelo LPN, Araújo JM, Pereiro AB, Archer M (2017) Fluorinated ionic liquids for protein drug delivery systems: investigating their impact on the structure and function of lysozyme. Int J Pharm 526:309–320
Arakawa T, Kita Y, Timasheff SN (2007) Protein precipitation and denaturation by dimethyl sulfoxide. Biophys Chem 131:62–70
Blake C, Koenig D, Mair G, North A, Phillips D, Sarma V (1965) Structure of hen egg-white lysozyme: a three-dimensional Fourier synthesis at 2 Å resolution. Nature 206:757
Blumlein A, McManus JJ (2013) Reversible and non-reversible thermal denaturation of lysozyme with varying pH at low ionic strength. BBA-Proteins Proteom 1834:2064–2070
Böhm G, Muhr R, Jaenicke R (1992) Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng, Des Sel 5:191–195
Bye JW, Falconer RJ (2013) Thermal stability of lysozyme as a function of ion concentration: a reappraisal of the relationship between the Hofmeister series and protein stability. Protein Sci 22:1563–1570
Cao A, Hu D, Lai L (2004) Formation of amyloid fibrils from fully reduced hen egg white lysozyme. Protein Sci 13:319–324
Cohn EJ et al (1950) A System for the separation of the components of human blood: quantitative procedures for the separation of the protein components of human plasma 1a, b, c. J Am Chem Soc 72:465–474
Day L, Zhai J, Xu M, Jones NC, Hoffmann SV, Wooster TJ (2014) Conformational changes of globular proteins adsorbed at oil-in-water emulsion interfaces examined by synchrotron radiation circular dichroism. Food Hydrocolloids 34:78–87
dos Santos R, Carvalho AL, Roque ACA (2017) Renaissance of protein crystallization and precipitation in biopharmaceuticals purification. Biotechnol Adv 35:41–50
Englard S, Seifter S (1990) Precipitation techniques. Methods Enzymol 182:285–300
Epstein PS, Plesset MS (1950) On the stability of gas bubbles in liquid-gas solutions. J Chem Phys 18:1505–1509
Gaul DA, Rickard DL, Needham D (2014) Microglassificationtm: a novel technique for protein dehydration. J Pharm Sci 103:810–820
Gaul DA, Bitterfield DL, Su JT, Li VM, Singh I, Morton J, Needham D (2015) Enzyme dehydration using microglassification™ preserves the protein's structure and function. J Pharm Sci 104:640–651
Giteau A et al (2008) Reversible protein precipitation to ensure stability during encapsulation within PLGA microspheres. Eur J Pharm Biopharm 70:127–136
Golunski S, Astolfi V, Carniel N, de Oliveira D, Di Luccio M, Mazutti MA, Treichel H (2011) Ethanol precipitation and ultrafiltration of inulinases from Kluyveromyces marxianus. Sep Purif Technol 78:261–265
Griebenow K, Klibanov AM (1995) Lyophilization-induced reversible changes in the secondary structure of proteins. Proc Natl Acad Sci 92:10969–10976
Hammerschmidt N, Hintersteiner B, Lingg N, Jungbauer A (2015) Continuous precipitation of IgG from CHO cell culture supernatant in a tubular reactor. Biotechnol J 10:1196–1205
Hammerschmidt N, Hobiger S, Jungbauer A (2016) Continuous polyethylene glycol precipitation of recombinant antibodies: sequential precipitation and resolubilization. Process Biochem 51:325–332
James S, McManus JJ (2012) Thermal and solution stability of lysozyme in the presence of sucrose, glucose, and trehalose. J Phys Chem B 116:10182–10188
Kang Y et al (2013) Development of a novel and efficient cell culture flocculation process using a stimulus responsive polymer to streamline antibody purification processes. Biotechnol Bioeng 110:2928–2937
Khan JM, Chaturvedi SK, Rahman SK, Ishtikhar M, Qadeer A, Ahmad E, Khan RH (2014) Protonation favors aggregation of lysozyme with SDS. Soft Matter 10:2591–2599
Kramer RM, Shende VR, Motl N, Pace CN, Scholtz JM (2012) Toward a molecular understanding of protein solubility: increased negative surface charge correlates with increased solubility. Biophys J 102:1907–1915
Krebs MR et al (2000) Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the β-domain. J Mol Biol 300:541–549
Kuehner DE, Heyer C, Rämsch C, Fornefeld UM, Blanch HW, Prausnitz JM (1997) Interactions of lysozyme in concentrated electrolyte solutions from dynamic light-scattering measurements. Biophys J 73:3211–3224
Kulkarni SS, Suryanarayanan R, Rinella Jr JV, Bogner RH (2018) Mechanisms by which crystalline mannitol improves the reconstitution time of high concentration lyophilized protein formulations. Eur J Pharm Biopharm 131:70–81
Kundu S, Aswal V, Kohlbrecher J (2017) Effect of ethanol on structures and interactions among globular proteins. Chem Phys Lett 670:71–76
Kurinomaru T, Shiraki K (2017) Aggregative protein–polyelectrolyte complex for high-concentration formulation of protein drugs. Int J Biol Macromol 100:11–17
Matagne A, Dobson CM (1998) The folding process of hen lysozyme: a perspective from the ‘new view’. Cell Mol Life Sci 54:363–371
Matsuda A, Mimura M, Maruyama T, Kurinomaru T, Shiuhei M, Shiraki K (2018) Liquid droplet of protein-polyelectrolyte complex for high-concentration formulations. J Pharm Sci 107:2713–2719
McPherson A, Gavira JA (2014) Introduction to protein crystallization. Acta Crystallogr, Sect F: Struct Biol Commun 70:2–20
Radomski KU, Lattner G, Schmidt T, Römisch J (2017) Pathogen safety of a new intravenous immune globulin 10% liquid. BioDrugs 31:125–134
Rosa PA, Azevedo AM, Sommerfeld S, Mutter M, Bäcker W, Aires-Barros MR (2013) Continuous purification of antibodies from cell culture supernatant with aqueous two-phase systems: from concept to process. Biotechnol J 8:352–362
Shire SJ, Shahrokh Z, Liu J (2004) Challenges in the development of high protein concentration formulations. J Pharm Sci 93:1390–1402
Singh N, Arunkumar A, Chollangi S, Tan ZG, Borys M, Li ZJ (2016) Clarification technologies for monoclonal antibody manufacturing processes: Current state and future perspectives. Biotechnol Bioeng 113:698–716
Sommer R, Satzer P, Tscheliessnig A, Schulz H, Helk B, Jungbauer A (2014) Combined polyethylene glycol and CaCl2 precipitation for the capture and purification of recombinant antibodies. Process Biochem 49:2001–2009
Sommer R, Tscheliessnig A, Satzer P, Schulz H, Helk B, Jungbauer A (2015) Capture and intermediate purification of recombinant antibodies with combined precipitation methods. BiochemEng J 93:200–211
Tanaka S, Oda Y, Ataka M, Onuma K, Fujiwara S, Yonezawa Y (2001) Denaturation and aggregation of hen egg lysozyme in aqueous ethanol solution studied by dynamic light scattering. Biopolymers 59:370–379
Tobler SA, Sherman NE, Fernandez EJ (2000) Tracking lysozyme unfolding during salt-induced precipitation with hydrogen exchange and mass spectrometry. Biotechnol Bioeng 71:194–207
van de Weert M, Hoechstetter J, Hennink WE, Crommelin DJA (2000) The effect of a water/organic solvent interface on the structural stability of lysozyme. J Controll Release 68:351–359
Vermeer AWP, Bremer MGEG, Norde W (1998) Structural changes of IgG induced by heat treatment and by adsorption onto a hydrophobic Teflon surface studied by circular dichroism spectroscopy. BBA-Gen Subj 1425:1–12
Voets IK, Cruz WA, Moitzi C, Lindner P, Arêas EP, Schurtenberger P (2010) DMSO-induced denaturation of hen egg white lysozyme. J Phys Chem B 114:11875–11883
Yonezawa Y, Tanaka S, Kubota T, Wakabayashi K, Yutani K, Fujiwara S (2002) An insight into the pathway of the amyloid fibril formation of hen egg white lysozyme obtained from a small-angle X-ray and neutron scattering study. J Mol Biol 323:237–251
Yoshikawa H, Hirano A, Arakawa T, Shiraki K (2012) Mechanistic insights into protein precipitation by alcohol. Int J Biol Macromol 50:865–871
Acknowledgements
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIT) (NRF-2018R1D1A1B07045154) and by the Technology Innovation Program (20000265, Stabilization platform of high concentration and stable liquid injection based on physical properties of biomaterials) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, D.G., Lee, J.C., Kim, D.J. et al. Effects of precipitation process on the biophysical properties of highly concentrated proteins. J. Pharm. Investig. 50, 493–503 (2020). https://doi.org/10.1007/s40005-020-00471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-020-00471-2