Abstract
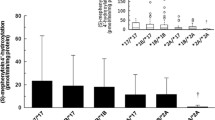
Nabumetone is a prodrug, used as an anti-inflammation agent and having the active metabolite 6-methoxy-2-naphthylacetic acid (6-MNA). The role of the polymorphic enzyme responsible for the 6-O-demethylation of 6-MNA to 6-hydroxy-2-naphtylacetic acid (6-HNA) was studied using recombinant cytochrome CYP2C9 microsomes (CYP2C9.1, CYP2C9.2 and CYP2C9.3) and human liver microsomes of known genotypes of CYP2C9. Utilizing recombinant CYP2C9.1, Vmax and Vmax/Km values of 6.3 ± 3.3 pmol/min/pmol P450 and 12.4 ± 4.7 nL/min/pmol P450, respectively, were obtained for the 6-MNA metabolism, and were almost similar to those in CYP2C9.2. In contrast, the Vmax/Km value in recombinant CYP2C9.3 was about one-third that of CYP2C9.1. In kinetic studies using liver microsomes of humans genotyped for the CYP2C9 genes, a sample genotyped as *3/*3 revealed about 4- to sixfold lower intrinsic clearance for 6-HNA formation than did samples genotyped as *1/*1. No appreciable differences were observed in kinetic parameters for 6-HNA formation in *1/*2 and *1/*3, while *2/*2 microsomes was comparable to wild type microsomes. In addition, S-warfarin 7-hydroxylation by recombinant CYP2C9.1 and CYP2C9.3 was inhibited by 6-MNA in a mixed manner. The apparent Ki value of 6-MNA on S-warfarin 7-hydroxylation by CYP2C9.3 was higher than that by CYP2C9.1. These results may provide valuable information for optimizing the anticoagulant activity of warfarin when nabumetone is co-administrated to patients.





Similar content being viewed by others
References
Crespi CL, Miller VP (1997) The R144C change in the CYP2C9*2 allele alters interaction of the cytochrome P450 with NADPH: cytochrome P450 oxidoreductase. Pharmacogenetics 7:203–210
Davies NM (1997) Clinical Pharmacokinetics of nabumetone. The dawn of selective cyclo-oxygenase-2 inhibition? Clin Pharmacokinet 33:403–416
Dennis VC, Thomas BK, Hanlon JE (2000) Potentiation of oral anticoagulation and hemarthrosis associated with nabumetone. Pharmacotherapy 20:234–239
Hanatani T, Fukuda T, Onishi S, Funae Y, Azuma J (2003) No major difference in inhibitory susceptibility between CYP2C9.1 and CYP2C9.3. Eur J Clin Pharmacol 59:233–235
Iida I, Miyata A, Arai M, Hirota M, Akimoto M, Higuchi S, Kobayashi K, Chiba K (2004) Catalytic roles of CYP2C9 and its variants (CYP2C9*2 and CYP2C9*3) in lornoxicam 5′-hydroxylation. Drug Metab Dispos 32:7–9
Iwakawa S, Miyashita K, Hashimoto Y, Kuroda T (2006) Effect of glimepiride and glibenclamide on S-warfarin 7-hydroxylation by human liver microsomes, recombinant human CYP2C9.1 and CYP2C9.3. Biol Pharm Bull 29:1983–1985
Iwatsubo T, Hirota N, Ooie T, Suzuki H, Shimada N, Chiba K, Ishizaki T, Green CE, Tyson CA, Sugiyama Y (1997) Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther 73:147–171
Jin Y, Borell H, Gardin A, Ufer M, Huth F, Camenisch G (2018) In vitro studies and in silico predictions of fluconazole and CYP2C9 genetic polymorphism impact on siponimod metabolism and pharamacokinetics. Eur J Clin Pharmacol 74:455–464
Kan T (2006) A book of statistical analysis for excel statistics (in Japanese). Esumi, Tokyo
Kirchheiner J, Brockmöller J (2005) Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin Pharmacol Ther 77:1–16
Kirchheiner J, Stormer E, Meisel C, Steinbach N, Roots I, Brockmoller J (2003) Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics 13:473–480
Kumar V, Wahlstrom JL, Rock DA, Warren CJ, Gorman LA, Tracy TS (2006) CYP2C9 inhibition: impact of probe selection and pharmacogenetics on in vitro inhibition profiles. Drug Metab Dispos 34:1966–1975
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12:251–263
Lewis DFV (2002) Homology modeling of human CYP2C family enzymes based on the CYP2C5 crystal structure. Xenobiotica 32:305–323
Mangan FR, Flack JD, Jackson D (1987) Preclinical overview of nabumetone. Pharmacology, bioavailability, metabolism, and toxicology. Am J Med 83:6–10
Matsumoto K, Nemoto E, Hasegawa T, Akimoto M, Sugibayashi K (2011) In vitro characterization of the cytochrome P450 isoforms involved in the metabolism of 6-methoxy-2-naphthylacetic acid, an active metabolite of the prodrug nabumetone. Biol Pharm Bull 34:734–739
Mikami E, Goto T, Ohno T, Matsumoto H, Nishida M (2000) Simultaneous analysis of naproxen, nabumetone and its major metabolite 6-methoxy-2-naphthylacetic acid in pharmaceuticals and human urine by high-performance liquid chromatography. J Pharm Biomed Anal 23:917–925
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Miners JO, Smith KJ, Robson RA, McManus ME, Veronese ME, Birkett M (1988) Tolbutamide hydroxylation by human liver microsomes. Kinetic characterization and relationship to other cytochrome P-450 dependent xenobiotic oxidations. Biochem Pharmacol 37:1137–1144
Miners JO, Coulter S, Birkett DJ, Goldstein JA (2000) Torsemide metabolism by CYP2C9 variants and other human CYP2C subfamily enzymes. Pharmacogenetics 10:267–270
Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 4:39–42
Rodrigues AD, Roberts EM (1997) The in vitro interaction of dexmedetomidine with human liver microsomal cytochrome P4502D6 (CYP2D6). Drug Metab Dispos 25:651–655
Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, Miners JO, Birkett DJ, Goldstein JA (1996) The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 6:341–349
Světlík S, Štícha M, Matoušková O, Nespěšná L, Sklenář Z, Bartůněk A, Perlík F, Slanař O (2016) Nabumetone and 6-MNA pharmacokinetics, assessment of intrasubject variability and gender effect. Am J Ther 23:1498–1503
Tang C, Shou M, Rushmore TH, Mei Q, Sandhu P, Woolf EJ, Rose MJ, Gelmann A, Greenberg HE, De Lepeleire I, Van Hecken A, De Schepper PJ, Ebel DL, Schwartz JI, Rodriques AD (2001) In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by alleic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics. Pharmacogenetics 11:223–235
Terakawa M, Kuwahara N, Tsuchiya T, Ishibashi K, Noguchi H, Azuma J, Sawamura A, Kurimoto T (1988) Steady-state pharmacokinetics of oral nabumetone in man. Drug Metab Pharmacokinet 3:407–416
Tsuda-Tsukamoto M, Ogasawara Y, Kume T (2005) Role of human liver cytochrome P450 2C9 in the metabolism of a novel α4β1/α4β7 dual antagonist, TR-14035. Drug Metab Pharamacokinet 20:127–134
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T (1981) A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn 4:879–885
Yamaori S, Takami K, Shiozawa A, Sakuyama K, Matsuzawa N, Ohmori S (2015) In vitro inhibition of CYP2C9-mediated warfarin 7-hydroxylation by iguratimod: possible mechanism of iguratimod-warfarin interaction. Biol Pharm Bull 38:441–447
Yamazaki H, Shimada T (1997) Human liver cytochrome P450 enzymes involved in the 7-hydroxylation of R- and S-warfarin enantiomers. Biochem Pharmacol 54:1195–1203
Yasar U, Eliasson E, Forslund-Bergengren C, Tybring G, Gadd M, Sjöqvist F, Dahl ML (2001) The role of CYP2C9 genotype in the metabolism of diclofenac in vivo and in vitro. Eur J Clin Pharmacol 57:729–735
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflicts of interest or any financial ties to disclose.
Ethical standards
This work complies with all ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsumoto, K., Hasegawa, T., Ohara, K. et al. Roles of CYP2C9 and its variants (CYP2C9*2 and CYP2C9*3) in the metabolism of 6-methoxy-2-napthylacetic acid, an active metabolite of the prodrug nabumetone. J. Pharm. Investig. 50, 71–79 (2020). https://doi.org/10.1007/s40005-019-00428-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-019-00428-0