Abstract
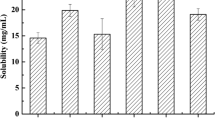
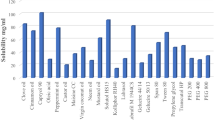
The objective was to study the phase behavior of different formulations, to identify the micro emulsion area and to prepare solid SMEDDS particles (SSMED) of loratadine (LOR) for improving oral bioavailability (BA). Formulations were prepared with either Lauroglycol 90 or Peceol as oils, Solutol HS15 as surfactant and Transcutol P as co-surfactant. Then water titrations were done to know the phase behavior to identify microemulsion zone. The SSMEDs were prepared by physical adsorption on novel silicon based porous particulate carriers. The flow properties of SSMEDs were studied. SSMED with syloid XDP (SSMED-XS) was optimized based on the free flowing nature. The optimized formulation was evaluated for self microemulsifying efficiency, size, PDI, Zeta potential (ZP), robustness in different pH conditions, surface morphology and in vitro release studies. Further, the pharmacokinetic (PK) behavior of SSMEDs was evaluated in wistar rats. The optimized L-SMEDDS formulation possessed a mean globule size of 140.90 ± 2.01 nm. Stearyl amine at 1% level was added as positive charge inducer. The PXRD and SEM studies indicated loss of crystallinity of the drug. In vitro % drug release for LOR powder was 65% and for L and SSMED was ranging in between 95–99% in 2 h. The BA of SSMED with stearyl amine (SSMED-XS) was 2.28 fold (p < 0.05); S-SMEDDS without stearyl amine (SSMED-X) was 1.78 fold (p < 0.001) more, when compared with plain LOR suspension. Cationic SSMEDs showed significant difference over negatively charged SSMED during in vivo studies. The SSMED-XS was found to be stable upto 3 months at room temperature (RT).





Similar content being viewed by others
References
Agarwal V, Siddiqui A, Ali H, Nazzal S (2009) Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS). Int J Pharm 366:44–52
Bahl D, Bogner RH (2006) Amorphization of indomethacin by co-grinding with Neusilin US2: amorphization kinetics, physical stability and mechanism. Pharm Res 23:2317–2325
Baird JA, Taylor LS (2010) Evaluation and modeling of the eutectic composition of various drug-polyethylene glycol solid dispersions. Pharm Dev Technol 16:201–211
Bernard A, Carlier H (1991) Absorption and intestinal catabolism of fatty acids in the rat: effect of chain length and unsaturation. Exp Physiol 76:445–455
Bloom B, Chaikoff IL, Reinhardt (1951) Intestinal lymph as pathway for transport of absorbed fatty acids of different chain lengths. Am J Physiol 166:451–455
Caliph SM, Charman WN, Porter CJ (2000) Effect of short, medium, and long chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci 89:1073–1084
Dixit RP, Nagarsenker MS (2008) Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci 35:183–192
Dudhipala N, Veerabrahma K (2015) Pharmacokinetic and pharmacodynamic studies of nisoldipine-loaded solid lipid nanoparticles developed by central composite design. Drug Dev Ind Pharm 41:1968–1977
El-Shabouri MH (2002) Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int J Pharm 249:101–108
Fabio C, Elisabetta C Pharmaceutical composition comprising a water/oil/water double microemulsion incorporated in a solid support. US20040247625 A1 9/8/2001
Gattefossé SA (1994) Microemulsions: formulation guide. Publication no. PF9225 A, Saint-Priest Cedex, France
Gershanik T, Benita S (1996) Positively charged self-emulsifying oil formulation for improving oral bioavailability of progesterone. Pharm Dev Technol 1:147–157
Gershanik T, Benzeno S, Benita S (1998) Interaction of the self-emulsifying lipid drug delivery system with mucosa of everted rat intestine as a function of surface charge and droplet size. Pharm Res 15:863–869
Ito Y, Kusawake T, Ishida M, Tawa R, Shibata N, Takada K (2005) Oral solid gentamicin preparation using emulsifier and adsorbent. J Control Release 105:23–31
Jain V, Prasad V, Jadhav P, Mishra PR (2009) Preparation and performance evaluation of saquinavir laden cationic submicron emulsions. Drug Deliv 16:37–44
Jakki R, Afzal Syed M, Kandadi P, Veerabrahma K (2013) Development of a self-microemulsifying drug delivery system of domperidone: in vitro and in vivo characterization. Acta Pharm 63:241–251
Janga KY, Jukanti R, Sunkavalli S, Velpula A, Bandari S (2012) In situ absorption and relative bioavailability studies of zaleplon loaded self nanoemulsifying powders. J Microencapsul 30:161–172
Kanika S, Yogesh P, Bansal AK (2010) Self emulsifying drug delivery systems: a strategy to improve oral bioavailability. CRIPS 11:42–49
Katteboina S, Chandrasekhar PVSR, Balaji S (2009) Approaches for the development of solid self emulsifying drug delivery systems and dosage forms. Asian J Pharm Sci 4:240–253
Kay GG, Harris AG (1999) Loratadine: a non-sedating antihistamine. Review of its effects on cognition, psychomotor performance, mood and sedation. Clin Expert Allergy 29:147–150
Kim DW, Kwon MS, Yousaf AM, Balakrishnan P, Park JH, Kim DS (2014) Comparison of a solid SMEDDS and solid dispersion for enhanced stability and bioavailability of clopidogrel napadisilate. Carbohydr Polym 114:365–374
Krishna Reddy KVSR, Moses Babu J, Ravindra Kumar Y, Vishnu Vardhan Reddy S, Kishore Kumar M (2003) Impurity profile study of loratadine. J Pharm Biomed Anal 32:29–39
Lipinski C (2002) Poor aqueous solubility-an industry wide problem in drug discovery. Am Pharm Rev 5:82–85
Neha K, Loveleen K, Rajni B, Manju N (2013) Recent updates on self micro emulsifying drug delivery systems. Am J PharmTech Res 3:261–288
Nekkanti V, Karatgi P, Prabhu R, Pillai R (2011) Solid self-microemulsifying formulation for candesartan cilexetil. AAPS PharmSciTech 11:9–17
Neslihan Gursoy R, Benita S (2004) Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother 58:173–182
Oh DH, Kang JH, Kim DW, Lee BJ, Kim JO, Yong CS, Choi HG (2011) Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm 420:412–418
Palmer AM (2003) New horizons in drug metabolism, pharmacokinetics and drug discovery. Drug News Perspect 16:57–62
Patel AR, Vavia PR (2007) Preparation and in-vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J 9:344–352
Pouton CW (2000) Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci 11:93–98
Pradeep P, Anant P (2006) Porous polystyrene beads as carriers for self-emulsifying system containing loratadine. AAPS PharmSciTech 7:1–7
Samridhi V, Sandeep Kumar S, Priya Ranjan Prasad V (2016) Solidified SNEDDS of loratadine: formulation using hydrophilic and hydrophobic grades of Aerosil, pharmacokinetic evaluations and in vivo–in silico predictions using GastroPlus. RSC Adv 6:3099–3116
Shailaja M, Diwan PV, Ramakrishna S, Madhusudan Rao Y (2013) Formulation optimization by box behnken statistical design and characterization of saquinavir loaded slns for brain delivery. IAJPR 3:1209–1224
Shashi M, Kamla P (2008) Formulation and evaluation of oil entrapped gastroretentive floating gel beads of loratadine. Acta Pharm 58:187–197
Shen H, Zhong M (2006) Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J Pharm Pharmacol 58:1183–1191
Sherafudeen SP, Vasantha PV (2015) Development and evaluation of in situ nasal gel formulations of loratadine. Res Pharm Sci 10:466–476
Shozan Mondal MD, Tauhidu Islam MD, Sharifur Rahman MD, Ariful Islam MD (2013) Dissolution enhancement of loratadine by formulating oleic acid and cremophor EL based self emulsifying drug delivery system (SEDDS). J App Pharm Sci 3:64–67
Swapna M, Afzal SM, Kishan V (2015) Cationic diclofenac lipid nanoemulsions for improved oral bioavailability: preparation, characterization and invivo evaluation. IJPSN 8:2874–2880
Tang B, Cheng G, Gu JC, Xu CH (2008) Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov Today 13:606–612
Tarate B, Chavan R, Bansal AK (2014) Oral solid self emulsifying formulations: a patent review. Recent Pat Drug Deliv Formul 8:126–143
Tayel SA, Soliman II, Louis D (2008) Improvement of dissolution properties of carbamazepine through application of the liquisolid tablet technique. Eur J Pharm Biopharm 69:342–347
Venkateshwarlu I, Prabhakar K, Ali M, Kishan V (2010) Development and in vitro cytotoxic evaluation of parenteral docetaxel lipid nanoemulsions for application in cancer treatment. PDA J Pharm Sci Technol 64:233–241
Villasmil-Sánchez S, Drhimeur W, Ospino SC, Rabasco Alvarez AM, González-Rodríguez ML (2010) Positively and negatively charged liposomes as carriers for transdermal delivery of sumatriptan: in vitro characterization. Drug Dev Ind Pharm 36:666–675
Yoshihara E, Nakae T (1986) Cytolytic activity of liposomes containing stearylamine. Biochim Biophys Acta Biomembr 854:93–101
Acknowledgements
One of the authors Mr. Katla Venu Madhav acknowledges AICTE, New Delhi, India for QIP fellowship to carry out this research work. The authors are grateful to M/s Aurobindo Pharma, Hyderabad for providing gift sample of loratadine. The authors are also grateful to M/s Abitec Corporation, USA and M/s Gattefosse, France for the generous gift samples of oils and surfactants. The authors acknowledge the help of M/s Fuji Chemicals, Japan for gift samples of Neusilin US2, Sylysia, M/s Grace & Co for gift samples of Syloid XDP, Syloid 244FP and M/s ACE capsules, Mumbai for gift samples of hard gelatin capsules. The funding was provided by All India Council for Technical Education (Grant No. PHR/724/12). The authors report no conflict of interest. The authors are alone responsible for the content and writing of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhav, K.V., Kishan, V. Self microemulsifying particles of loratadine for improved oral bioavailability: preparation, characterization and in vivo evaluation. J. Pharm. Investig. 48, 497–508 (2018). https://doi.org/10.1007/s40005-017-0344-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-017-0344-6