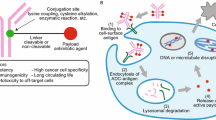
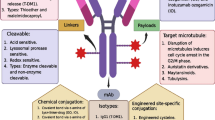
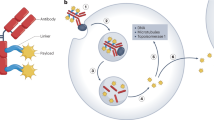
Abstract
Antibody–drug conjugates (ADCs) are tumor-targeted therapeutic agents that combine the specificity of monoclonal antibodies (mAbs) with the potent anti-tumor effects of cytotoxic drugs. Over the past few years, ADCs have become a powerful tool in the field of cancer chemotherapy. Recently, two ADC products, brentuximab vedotin (Adcetris®) and trastuzumab emtansine (Kadcyla®), have received FDA approval and there are more than 40 ADC candidates in clinical trials for the treatment of various cancers. Despite the success of some products, considerable interests for the next generation of ADCs have focused on the development of homogeneous conjugates because most of the current ADCs are highly heterogeneous with different drug-to-antibody ratios and drug conjugation sites. Recent studies have demonstrated that the site-specific conjugation of drugs to mAbs could produce homogeneous ADC with better pharmacokinetic properties and improved therapeutic index. A number of approaches, including the use of engineered cysteines, the insertion of unnatural amino acids, and enzymatic ligation, have addressed the challenging issues for the synthesis of homogeneous ADC. This review discusses the limitations of current ADC technologies and describes recent site-specific conjugation methods that can be used to prepare homogeneous ADCs for targeted anticancer drug delivery.



Similar content being viewed by others
References
Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E, Gisselbrecht C, Ketterer N, Nasta S, Rohatiner A, Schmidt-Wolf IG, Schuler M, Sierra J, Smith MR, Verhoef G, Winter JN, Boni J, Vandendries E, Shapiro M, Fayad L (2010) Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol 28:2085–2093
Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, Senter PD (2008) Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem 19:759–765
Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG (2012) Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci USA 109:16101–16106
Barok M, Tanner M, Koninki K, Isola J (2011) Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res 13:R46
Behrens CR, Liu B (2014) Methods for site-specific drug conjugation to antibodies. MAbs 6:46–53
Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R (2001) Approval summary gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 7:1490–1496
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, Yin JA, Hunter A, Goldstone AH, Wheatley K (2011) Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 29:369–377
Chudasama V, Maruani A, Caddick S (2016) Recent advances in the construction of antibody-drug conjugates. Nat Chem 8:114–119
DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, Kunz A, Hamann PR, Gorovits B, Udata C, Moran JK, Popplewell AG, Stephens S, Frost P, Damle NK (2004) Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 103:1807–1814
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778–784
Drake PM, Albers AE, Baker J, Banas S, Barfield RM, Bhat AS, de Hart GW, Garofalo AW, Holder P, Jones LC, Kudirka R, McFarland J, Zmolek W, Rabuka D (2014) Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjug Chem 25:1331–1341
Ducry L, Stump B (2009) Antibody–drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem 21:5–13
Feld J, Barta SK, Schinke C, Braunschweig I, Zhou Y, Verma A (2013) Linked-in: design and efficacy of antibody drug conjugates in oncology. Oncotarget 4:397–412
Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, Burris Iii HA, LoRusso PM, Yi JH, Saad O, Tong B, Chu YW, Holden S, Joshi A (2012) Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody–drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 69:1229–1240
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R, Hallett W, Tsou HR, Upeslacis J, Shochat D, Mountain A, Flowers DA, Bernstein I (2002) Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem 13:47–58
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 10:7063–7070
Harding FA, Stickler MM, Razo J, DuBridge R (2010) The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs 2:256–265
Hofer T, Skeffington LR, Chapman CM, Rader C (2009) Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry 48:12047–12057
Jeger S, Zimmermann K, Blanc A, Grunberg J, Honer M, Hunziker P, Struthers H, Schibli R (2010) Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem Int Ed Engl 49:9995–9997
Junutula JR, Bhakta S, Raab H, Ervin KE, Eigenbrot C, Vandlen R, Scheller RH, Lowman HB (2008a) Rapid identification of reactive cysteine residues for site-specific labeling of antibody-Fabs. J Immunol Methods 332:41–52
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, Lu Y, Meng YG, Ng C, Yang J, Lee CC, Duenas E, Gorrell J, Katta V, Kim A, McDorman K, Flagella K, Venook R, Ross S, Spencer SD, Lee Wong W, Lowman HB, Vandlen R, Sliwkowski MX, Scheller RH, Polakis P, Mallet W (2008b) Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 26:925–932
Katz J, Janik JE, Younes A (2011) Brentuximab vedotin (SGN-35). Clin Cancer Res 17:6428–6436
Kim EG, Kim KM (2015) Strategies and advancement in antibody–drug conjugate optimization for targeted cancer therapeutics. Biomol Ther 23:493–509
Kitson SL, Quinn DJ, Moody TS, Speed D, Watters W, Rozzell D (2013) Antibody–drug conjugates (ADCs)—biotherapeutic bullets. Chim Oggi-Chem Today 31:30–36
Kubota T, Niwa R, Satoh M, Akinaga S, Shitara K, Hanai N (2009) Engineered therapeutic antibodies with improved effector functions. Cancer Sci 100:1566–1572
Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, Stone I, Ryan MC, Sussman D, Lyon RP, Zeng W, Harrington KH, Klussman K, Westendorf L, Meyer D, Bernstein ID, Senter PD, Benjamin DR, Drachman JG, McEarchern JA (2013) SGN-CD33A: a novel CD33-targeting antibody–drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 122:1455–1463
Li X, Yang J, Rader C (2014) Antibody conjugation via one and two C-terminal selenocysteines. Methods 65:133–138
McCombs JR, Owen SC (2015) Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J 17:339–351
McDonagh CF, Turcott E, Westendorf L, Webster JB, Alley SC, Kim K, Andreyka J, Stone I, Hamblett KJ, Francisco JA, Carter P (2006) Engineered antibody–drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel 19:299–307
Na DH, Park EJ, Kim MS, Lee HS, Lee KC (2012) Application of sodium dodecyl sulfate-capillary gel electrophoresis to the characterization of ricin A-chain immunotoxins. Chromatographia 75:679–683
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR (2014) Site-specific antibody drug conjugates for cancer therapy. MAbs 6:34–45
Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM (2014) Antibody–drug conjugates: current status and future directions. Drug Discov Today 19:869–881
Peters C, Brown S (2015) Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep 35:e00225
Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Yang Y, Sievers EL, Kennedy DA, Shustov A (2012) Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 30:2190–2196
Rabuka D, Rush JS, Wu P, Bertozzi CR (2012) Site-specific chemical protein conjugation using genetically encoded aldehyde tags. Nat Protoc 7:1052–1067
Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC (2005) Monoclonal antibody successes in the clinic. Nat Protoc 23:1073–1078
Ricart AD (2011) Antibody–drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res 17:6417–6427
Riechmann L, Clark M, Waldmann H, Winter G (1988) Reshaping human antibodies for therapy. Nature 332:323–327
Schroff RW, Foon KA, Beatty SM, Oldham RK, Morgan AC (1985) Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res 45:879–885
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12:278–287
Senter PD, Sievers EL (2012) The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol 30:631–637
Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, Li D, Tibbitts J, Baudys J, Saad OM, Scales SJ, McDonald PJ, Hass PE, Eigenbrot C, Nguyen T, Solis WA, Fuji RN, Flagella KM, Patel D, Spencer SD, Khawli LA, Ebens A, Wong WL, Vandlen R, Kaur S, Sliwkowski MX, Scheller RH, Polakis P, Junutula JR (2012) Conjugation site modulates the in vivo stability and therapeutic activity of antibody–drug conjugates. Nat Biotechnol 30:184–189
Sievers EL, Senter PD (2013) Antibody–drug conjugates in cancer therapy. Ann Rev Med 64:15–29
Sochaj AM, Swiderska KW, Otlewski J (2015) Current methods for the synthesis of homogeneous antibody–drug conjugates. Biotechnol Adv 33:775–784
Stimmel JB, Merrill BM, Kuyper LF, Moxham CP, Hutchins JT, Fling ME, Kull FC Jr (2000) Site-specific conjugation on serine right-arrow cysteine variant monoclonal antibodies. J Biol Chem 275:30445–30450
Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, Zhou D, Chandrasekaran R, Samain C, Loo C, Rossi A, Rickert M, Krimm S, Wong T, Chin SM, Yu J, Dilley J, Chaparro-Riggers J, Filzen GF, O’Donnell CJ, Wang F, Myers JS, Pons J, Shelton DL, Rajpal A (2013) Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol 20:161–167
Sukumaran S, Gadkar K, Zhang C, Bhakta S, Liu L, Xu K, Raab H, Yu SF, Mai E, Fourie-O’Donohue A, Kozak KR, Ramanujan S, Junutula JR, Lin K (2015) Mechanism-based pharmacokinetic/pharmacodynamic model for THIOMAB™ drug conjugates. Pharm Res 32:1884–1893
Sun MM, Beam KS, Cerveny CG, Hamblett KJ, Blackmore RS, Torgov MY, Handley FG, Ihle NC, Senter PD, Alley SC (2005) Reduction–alkylation strategies for the modification of specific monoclonal antibody disulfides. Bioconjug Chem 16:1282–1290
Takimoto JK, Adams KL, Xiang Z, Wang L (2009) Improving orthogonal tRNA-synthetase recognition for efficient unnatural amino acid incorporation and application in mammalian cells. Mol BioSyst 5:931–934
Teicher BA, Chari RV (2011) Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res 17:6389–6397
van der Velden VH, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, van Dongen JJ (2001) Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 97:3197–3204
Walker S, Landovitz R, Ding WD, Ellestad GA, Kahne D (1992) Cleavage behavior of calicheamicin gamma 1 and calicheamicin T. Proc Natl Acad Sci USA 89:4608–4612
Wang L, Amphlett G, Blättler WA, Lambert JM, Zhang W (2005) Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci 14:2436–2446
Yokoyama K, Nio N, Kikuchi Y (2004) Properties and applications of microbial transglutaminase. Appl Microbiol Biotechnol 64:447–454
Younes A, Yasothan U, Kirkpatrick P (2012) Brentuximab vedotin. Nat Rev Drug Discov 11:19–20
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (NRF-2013R1A2A2A01068858).
Conflict of interest
All authors (Y. Kim, E.J. Park, and D.H. Na) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Park, E.J. & Na, D.H. Antibody–drug conjugates for targeted anticancer drug delivery. Journal of Pharmaceutical Investigation 46, 341–349 (2016). https://doi.org/10.1007/s40005-016-0254-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0254-z