Abstract
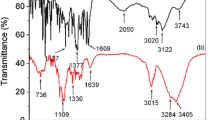
The objective of the present study was to prepare orodispersible tablets of rizatriptan benzoate using hydroxypropyl β cyclodextrin (HPβCD) to mask the bitter taste. Taste-masked complex of rizatriptan was prepared with HPβCD by kneading, and analyzed for drug content, FTIR, DSC and bitterness test. Tablets were prepared by direct compression using crospovidone as superdisintegrant. The tablets were evaluated for weight variation, mechanical strength, in vitro disintegration time, wetting time, water absorption ratio, and in vitro release characteristics. The complex showed effective taste masking as compared to plain drug. Hardness and friability data indicated good mechanical strength of tablets. The tablets dispersed rapidly in 35 to 71 s with faster release rate of rizatriptan benzoate. The stability studies for 3 months showed no significant changes in quality of tablets. Hence the present studies indicated the abilities of HPβCD for taste masking and improving the dissolution profile of rizatriptan benzoate after complexation.



Similar content being viewed by others
References
Albertini B, Cavallari C, Passerini N, Voinovich D, Rodriguez ML, Magarotto L, Rodriguez L (2004) Characterization and taste masking evaluation of acetaminophen granules: comparison between different preparation methods in a high shear mixer. Eur J Pharm Sci 21:295–303
Ammar HO, Salama HA, Ghorab M, Mahmoud AA (2006) Implication of inclusion complexation of glimepiride in cyclodextrin–polymer systems on its dissolution, stability and therapeutic efficacy. Int J Pharm 320:53–57
Banker GS, Anderson GR In: Lachman L, Leon Liberman HA, Kanig JL (Eds.) (1987) The theory and practice of industrial pharmacy. Varghese Publishing House, Mumbai, 293–45
Bi Y, Sunada H, Yonezawa Y, Danjo K, Lida K (1996) Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity. Chem Pharm Bull 44:2121–2127
Chang RK, Guo X, Burnside BA, Couch RA (2000) Fast dissolving tablets. Pharm Technol 246:52–58
Chaudhari PD, Chaudhari SP, Kolhe SR, Dave KV, More DM (2005) Formulation and evaluation of fast dissolving tablets of famotidine. Indian Drugs 42:641–649
Corveleyn S, Remon JP (1997) Formulation and production of rapidly disintegrating tablets by lyophilization using hydrochlorothiazide as a model drug. Int J Pharm 152:215–225
Gao Y, Cui F, Guan Y, Yang L, Wang Y, Zhang L (2006) Preparation of roxithromycin-polymeric microspheres by the emulsion solvent diffusion method for taste masking. Int J Pharm 318:62–69
Gohel MC, Patel M, Amin A, Agrawal R, Dave R, Bariya N (2004) Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS Pharm Sci Tech 5(36):1–6
Gohel MC, Parikh RK, Brahmbhatt BK, Shah AR (2007) Improving the tablet characteristics and dissolution profile of ibuprofen by using a novel coprocessed superdisintegrant: a technical note. AAPS Pharm Sci Tech 8(13):E1E6
Gosai AR, Patil SB, Sawant KK (2008) Formulation and evaluation of orodispersible tablets of ondansetron hydrochloride by direct compression using superdisintegrants. Int J Pharm Sci Nanotech 1(1):104–111
Haware RV, Chaudhari PD, Parakh SR, Bauer-Brandl A (2008) Development of a melting tablet containing promethazine HCl against motion sickness. AAPS Pharm Sci Tech 9(3):1006–1015
Indian Pharmacopeia (1996) Government of India, Ministry of Health and Family Welfare, Controller of Publication, Delhi
Lee CW, Kim SJ, Youn YS, Widjojkusumo E, Lee YH, Kim J, Lee YW, Tjandrawinata RR (2010) Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J Supercritical Fliuds 55:348–357
Madgulkar AR, Bhalekar MR, Padalkar RR (2009) Formulation design and optimization of novel taste masked mouth-dissolving tablets of tramadol having adequate mechanical strength. AAPS Pharm Sci Tech 10(2):574–581
Mielcarek J, Czernielewska A, Czarczynska B (2006) Inclusion complexes of felodipine and amlodipine with methylbeta-cyclodextrin. J Inclus Phenom Macro Chem 54:17–21
Pather SI, Khankari R, Siebert J (2005) Quick dissolving intraoral tablets. In: Ghosh TK, Pfister WR (eds) Drug delivery to the oral cavity: molecules to market. CRC Press, New York, NY, pp 291–336
Pharmacopeia Indian (2007) Government of India, Ministry of Health and Family Welfare. Indian Pharmacopoeial Commission, Ghaziabad
Schiermeier S, Schmidt PC (2002) Fast dispersible ibuprofen tablets. Eur J Pharm Sci 15:295–305
Setty CM, Prasad DVK, Gupta VRM (2008) Development of fast dispersible aceclofenac tablet: effect of functionality of superdisintegrants. Indian J Pharm Sci 70(2):180–185
Shah PP, Mashru RC (2008a) Development and evaluation of artemether taste masked rapid disintegrating tablets with improved dissolution using solid dispersion technique. AAPS Pharm Sci Tech 9(2):494–500
Shah PP, Mashru RC (2008b) Formulation and evaluation of taste masked oral reconstitutable suspension of primaquine phosphate. AAPS Pharm Sci Tech 9(3):1025–1030
Shimizu T, Kameoka N, Iki H, Tabata T, Hamaguchi N, Igari Y (2003a) Formulation study for lansoprazole fast disintegrating tablet. II. Effect of triethyl citrate on the quality of the products. Chem Pharm Bull 51(9):1029–1035
Shimizu T, Sugaya M, Nakano Y, Izutsu D, Mizukami Y, Okochi K, Tabata T, Hamaguchi N, Igari Y (2003b) Formulation study for lansoprazole fast- disintegrating tablet. III. Design of rapidly disintegrating tablets. Chem Pharm Bull 51(10):1121–1127
Shin C, Oh IJ, Lee YB, Choi HK, Choi JS (1998) Enhanced dissolution of furosemide by coprecipitating or cogrinding with crospovidone. Int J Pharm 175:17–24
Shirwaikar A (2004) Fast disintegrating tablets of atenolol by dry granulation method. Ind J Pharm Sci 66:422–426
Sohi H, Sultana Y, Khar RK (2004) Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm 30(5):429–448
Szejtli J, Szente L (2005) Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur J Pharm Biopharm 61:115–125
Tiwari G, Tiwari R, Awani K (2010) Cyclodextrins in drug delivery system: applications. J Pharm Bioall Sci 2(2):72–79
Yajima T, Umeki N, Itai S (1999) Optimum spray congealing conditions for masking the bitter taste of clarithromycin in wax matrix. Chem Pharm Bull 47:220–225
Zhao N, Augusburger L (2005) The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorthiazide from directly compressed tablets. AAPS Pharm Sci Tech 6(1):E120–E126
Acknowledgments
The authors are thankful to Dr. C. D. Upasani for providing facilities to carry out the research work. The authors are also thankful to Nosch Laboratories Pvt. Ltd., Medak, India; Gangwal Chemicals Pvt. Ltd., India and Gattefosse, St Priest, Cedex, France for providing gift of rizatriptan benzoate, HPβCD and Gelucire 43/01 respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (U. N. Dungarwal, S. B. Patil) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dungarwal, U.N., Patil, S.B. Development of orodispersible tablets of taste masked rizatriptan benzoate using hydroxypropyl β cyclodextrin. Journal of Pharmaceutical Investigation 46, 537–545 (2016). https://doi.org/10.1007/s40005-016-0240-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0240-5