Abstract
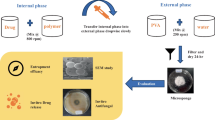
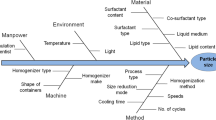
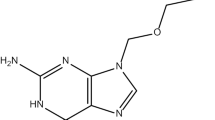
The aim of the present research was to develop Fluconazole loaded microsponge-based topical delivery system for controlled release and enhanced drug deposition in the skin. Microsponges containing fluconazole were prepared by an emulsion solvent diffusion method. The effect of formulation variables (drug: polymer ratio, internal phase volume and amount of emulsifier) and process variables (stirring time and stirring speed) on the physical characteristics of microsponges like Production yield, Mean particle size, Entrapment efficiency were investigated. The effect of internal phase volume and amount of emulsifier on the physical characteristics of microsponges were examined on optimized drug/polymer ratio, stirring speed and stirring time by 32 factorial design. The optimized microsponges were dispersed into a hydrogel and evaluated. In vitro drug release, Ex vivo drug deposition, primary skin irritancy study and In vivo antibacterial activity of fluconazole-loaded formulations were studied. Spherical and porous FLU microsponge particles were obtained. From 32 factorial design, it was concluded that optimized microsponge possess particle size, production yield and entrapment efficiency of 2.45 μm, 77.38 and 92.33 %, respectively. Microsponge-loaded gels demonstrated controlled release, no irritancy to rat skin and antifungal activity. An In vivo skin deposition study demonstrated four fold higher retention in the stratum corneum layer as compared with marketed cream. Microsponges-based gel formulations showed prolonged efficacy in mouse surgical wound model infected with Candida spp. Fluconazole was stable in topical formulations and showed enhanced retention in the skin indicating better potential of the delivery system for treatment of primary and secondary skin infections.












Similar content being viewed by others
References
Allen L, Popovich N, Ansel H, Ansel H (2005) Ansel’s pharmaceutical dosage forms and drug delivery systems. Lippincott Williams & Wilkins, Philadelphia
Amrutiya N, Bajaj A, Madan M (2009) Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech 10:402–409
Barbanoj M, Antonijoan R, Garca-a-Gea C, Puntes M, Gich I, Jana F (2005) Eberconazole cream: topical and general tolerability, sensitisation potential and systemic availability. Method Find Exp Clin 27:227–235
Barel A, Paye M, Maibach H (2005) Handbook of cosmetic science and technology, 2nd edn. Taylor and Francis, Hoboken
Behan N, O’Sullivan C, Birkinshaw C (2002) Synthesis and in-vitro drug release of insulin-loaded poly(n-butyl cyanoacrylate) nanoparticles. Macromol Biosci 2:336–340
Bothiraja C, Kapare H, Pawar A, Shaikh K (2013) Development of plumbagin-loaded phospholipid Tween 80 mixed micelles: formulation, optimization, effect on breast cancer cells and human blood/serum compatibility testing. Ther Deliv 4:1247–1259
Chadawar V, Shaji J (2007) Microsponge delivery system. CDD 4:123–129
Comoglu T, Gonul N, Baykara T (2003) Preparation and in vitro evaluation of modified release ketoprofen microsponges. II Farmaco 58:101–106
Espinel-Ingroff A (2009) Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005–2009). Rev Iberoam Micol 26:15–22
Garber G (2001) An overview of fungal infections. Drugs 61:1–12
Gennaro A (2000) Remington. Lippincott Williams & Wilkins, Baltimore
Gisby J, Bryant J (2000) Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob Agents Chemother 44:255–260
Gungor S, Erdal M, Aksu B (2013) New formulation strategies in topical antifungal therapy. JCDSA 03:56–65
Herwadkar A, Banga A (2012) An update on the application of physical technologies to enhance intradermal and transdermal drug delivery. Ther Deliv 3:339–355
Jain V, Jain D, Singh R (2010) Factors affecting the morphology of eudragit S-100 based microsponges bearing dicyclomine for colonic delivery. J Pharm Sci 100:1545–1552
Jelvehgari M, Siahi-Shadbad M, Azarmi S, Martin G, Nokhodchi A (2006) The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm 308:124–132
Kaity S, Isaac J, Ghosh A (2013) Interpenetrating polymer network of locust bean gum-poly (vinyl alcohol) for controlled release drug delivery. Carbohydr Polym 94:456–467
Kharb V, Saharan V, Dev K, Jadhav H, Purohit S (2014) Formulation, evaluation and 3 2 full factorial design-based optimization of ondansetron hydrochloride incorporated taste masked microspheres. Pharm Dev Technol 19:839–852
Lademann J, Jacobi U, Surber C, Weigmann H, Fluhr J (2009) The tape stripping procedure: evaluation of some critical parameters. Eur J Pharm Biopharm 72:317–323
Lesher J (1999) Oral therapy of common superficial fungal infections of the skin. J Am Acad Dermatol 40:S31–S34
Maheshwari R, Tekade R, Sharma P, Darwhekar G, Tyagi A, Patel R, Jain D (2012) Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi Pharm J 20:161–170
Maiti S, Kaity S, Ray S, Sa B (2011) Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm. doi:10.2478/v10007-011-0022-6
McRipley R, Whitney R (1976) Characterization and quantitation of experimental surgical-wound infections used to evaluate topical antibacterial agents. Antimicrob Agents Chemother 10:38–44
Mukerjee R, Wu C (2006) A modern theory of factorial design. Springer, New York
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515
Nokhodchi A, Jelvehgari M, Siahi M, Mozafari M (2007) Factors affecting the morphology of benzoyl peroxide microsponges. Micron 38:834–840
Orlu M, Cevher E, Araman A (2006) Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 318:103–117
Parfitt K, Martindale W (1999) Martindale. Pharmaceutical Press, London
Pawar A, Shelake M, Bothiraja C, Kamble R (2012) Development of photostable gastro retentive formulation for nifedipine using low-density polypropylene microporous particles. J Microencapsul 29:409–416
Sabyasachi M, Santanu K, Somasree R (2011) Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm 61:257–270
Shaikh K, Chellampillai B, Pawar A (2010) Studies on nonionic surfactant bilayer vesicles of ciclopirox olamine. Drug Dev Ind Pharm 36:946–953
Shukla R, Tiwari A (2012) Carbohydrate polymers: applications and recent advances in delivering drugs to the colon. Carbohydr Polym 88:399–416
Acknowledgments
All authors (N. Patel, N. Padia, N. Vadgama, M. Raval, N. Sheth) declare that they have no conflict of interest. The authors are thankful to Nectar Drugs Pvt Ltd, Mumbai and Asha cellulose, Baroda for providing gift sample of drug and polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, N., Padia, N., Vadgama, N. et al. Formulation and evaluation of microsponge gel for topical delivery of fluconazole for fungal therapy. Journal of Pharmaceutical Investigation 46, 221–238 (2016). https://doi.org/10.1007/s40005-016-0230-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0230-7