Abstract
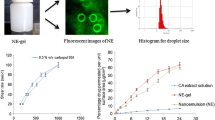
This study was aimed at designing mucoadhesive microemulsion gel to enhance the brain uptake of curcumin through intranasal route. Suitable oil, surfactant and cosurfactant for the development of microemulsion were selected based on maximum curcumin solubility, drug excipients compatibility through FTIR study and non-toxicity to sheep nasal mucosa. Curcumin loaded mucoadhesive microemulsion (CMME) was developed by incorporating polycarbophil as mucoadhesive polymer into Capmul MCM based optimal microemulsion (CME) and was subjected to characterization, stability, mucoadhesion and naso-ciliotoxicity study. Brain uptake of Curcumin via nasal route was studied by performing biodistribution study in Swiss albino rats. CME was found to be transparent, stable and non ciliotoxic with 57.66 nm ± 3.46, −16.28 mV ± 4.11 and 98.08 % ± 1.01 as average globule size, zeta potential and drug content respectively. PdI and TEM study depicted the narrow size distribution of CME. Following single intranasal administration of CMME and CME at dose of 2.86 mg/kg, Maximum Curcumin uptake in the olfactory bulb was more than 11 fold (51.1 ± 2.8) than that of intravenous injection of Curcumin solution (4.4 ± 1.1). AUC ratio of brain tissues to that in plasma obtained after nasal administration of CMME were significantly higher than those after intravenous administration of Curcumin solution. Findings of the present study revealed that optimal CMME and intranasal route may be considered to be promising and an alternative approach for brain targeting of Curcumin.







Similar content being viewed by others
References
Anant P, Anshuman AA, Bhimrao KJ, Mahadik KR (2004) Characterization of curcumin–PVP solid dispersion obtained by spray drying. Int J Pharm 271:281–286
Bachhav YG, Patravale VB (2009) Microemulsion based vaginal gel of clotrimazole: formulation, in vitro evaluation and stability studies. AAPS PharmSciTech 10:476–481
Bechgaard E, Gizurarson S, Hjortkjaer RK (1997) Pharmacokinetic and pharmacodynamic response after intranasal administration of diazepam to rabbits. J Phar Pharmacol 49:747–750
Behl CR, Pimplaskar HK, Sileno AP, Demeireles J, Romeo VD (1998) Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv drug deliver rev 29:89–116
Bittera C, Katja SZ, Surber C (2011) Nasal drug delivery in humans. Curr Probl Dermatol 40:20–35
Botner S, Sintov AC (2011) Intranasal delivery of two benzodiazepines, midazolam and diazepam, by a microemulsion system. Pharmacol Pharm 2:180–188
Bshara HN, Ahmed RO, Holayel SM, El-Shamy AA (2012) Improvement of the bioavailability of buspirone HCl using intranasal delivery systems. J Pharm Sci 45:86–102
Bshara H, Osman R, Mansour S, Abd El-Hameed A, El-Shamy AEHA (2014) Chitosan and cyclodextrin in intranasal microemulsion for improved brain buspirone hydrochloride pharmacokinetics in rats. Carbohydr Polym 99:297–305
Chen X, Zhi F, Zhang X, Ambardekar R, Meng Z, Paradkar AR, Hu Y, Yang Y (2013) Enhanced brain targeting of curcumin by intranasal administration of a thermosensitive poloxamer hydrogel. J Pharm Pharmacol 65(6):807–816
Dhuria SV, Hanson LR, Frey WH (2010) Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99:1654–1673
Elshafeey AH, Bendas ER, Mohamed OH (2009) Intranasal microemulsion of sildenafil citrate: in vitro evaluation and in vivo pharmacokinetic study in rabbits. AAPS PharmSciTech 10(2):361–367
Jeffrey P, Summerfield S (2010) Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol Dis 37:33–37
Joshi M, Patravale V (2006) Formulation and evaluation of nanostructured lipid carrier (NLC)-based gel of valdecoxib. Drug Dev Ind Pharm 32:911–918
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K (2008) Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 24:285–291
Lawrencea MJ, Reesb GD (2000) Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev 45:89–121
Li L, Nandi I, Kim KH (1998) Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of diazepam. Int J Pharm 237:77–85
Li J, Jiang Y, Wen J, fan G, Wu Y, Zhang C (2009) A rapid and simple HPLC method for the determination of curcumin in rat plasma: assay development, validation and application to a pharmacokinetic study of curcumin liposome. Biomed Chromatogr 23(11):1201–1207
Mandal S (2011) Microemulsion drug delivery system: design and development for oral bioavailability enhancement of lovastatin. S Afr Pharm J 78(3):44–50
Mandal S, Mandal SD (2010) Design and development of carbamazepine mucoadhesive microemulsion for intranasal delivery: an ex vivo study. IJPSRR 3:56–60
Mandal S, Mandal SD, Surti N, Patel VB (2009) Design and development of saquinavir microemulsion for the oral bioavailability enhancement. Int J PharmTech Res 1(4):1442–1448
Mandal S, Mandal SD, Surti N, Patel VB (2012) Development of microemulsion formulation for the solubility enhancement of flunarizine. Der Pharm Lett 2(3):227–236
Mandal SD, Mandal S, Patel J (2013) Development of curcumin loaded microemulsion drug delivery System for improving its dissolution profile. IJPFA 4(2):101–107
Mohmmahad S, Khan RA, Mustafa G, Chuttani K, Baboota S, Sahni JK, Ali J (2013) Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci 48(3):393–405
Pak Y, Patek R, Mayersohn M (2003) Sensitive and rapid isocratic liquid chromatography method for quantitation of curcumin in plasma. J Chromatogr B 796:339–346
Patel MB, Mandal S, Rajesh KS (2012) Formulation and kinetic modeling of curcumin loaded intranasal mucoadhesive microemulsion. J Pharm Bioallied Sci 4:81–83
Pathak R, Dash RP, Misra M, Nivsarkar M (2014) Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm Sin B 4:151–160
Pisal SS, Reddy P, Paradkar AR (2004) Nasal melatonin gels using pluronic PF-127 for chronobiological treatment of sleep disorder. Ind J Biotechnol 3:369–377
Rao J, Mcclements DJ (2011) Formation of flavor oil microemulsions, nanoemulsions and emulsions: influence of composition and preparation method. J Agric Food Chem 59(9):5026–5035
Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R (1995) Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 94:79–83
Shah B, Khunt D, Bhatt H, Misra M, Padh H (2015) Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parameters. Eur J Pharm Sci 78:54–66
Soni H (2011) Qualitative and quantitative profile of curcumin from ethanolic extract of curcuma longa. Int Res J Pharm 2(4):180–184
Stevens J, Ploeger BA, Graaf PH, Danhof M, Elizabeth CM (2011) Systemic and direct nose-to-brain transport pharmacokinetic model for remoxipride after intravenous and intranasal administration. Drug Metabo Dispos 39(12):2275–2282
Swamya NGN, Abbasb Z (2012) Mucoadhesive in situ gels as nasal drug delivery systems: an overview. Asian J Pharm Sci 7(3):168–180
Tauseef SM, Butt MS, Anjum FM (2009) Safety assessment of black cumin fixed and essential oil in normal Sprague Dawley rats: serological and hematological indices. Food Chem Toxicol 47:2768–2775
Tenjarla SN (1999) Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carr Syst 16:461–521
Tian XH, Lin XN, Wei F (2011) Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomed 6:445–452
Tsai YM, Chiena CF, Lina LC, Tsai TH (2011) Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm 5(3):1–8
Tubesha Z, Mustapha UI, Mahmud R, Ismail M (2013) Study on the potential toxicity of a thymoquinone-rich fraction nanoemulsion in sprague dawley rats. Molecules 18:7460–7472
Vyas TK, Babbar AK, Sharma RK, Singh S, Misra AN (2006) Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech 7:E1–E6
Wang X, Chi N, Tang X (2008) Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm 70:735–740
Acknowledgments
All authors (S. D. Mandal, S. Mandal and J. Patel) declare that they have no confilct of interest. All authors are thankful to Arjuna Natural Extracts Pvt. Ltd., Hyderabad, India for providing Curcumin and Emodin as gift sample. The authors express their gratitude to ABITECH, Mumbai, India for providing oils and surfactants as gift sample. The authors are also grateful to Dr. Kavitha Pillai for proof-reading the manuscript for grammatical and spelling errors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandal, S.D., Mandal, S. & Patel, J. Brain targeting efficiency of Curcumin loaded mucoadhesive microemulsion through intranasal route. Journal of Pharmaceutical Investigation 46, 179–188 (2016). https://doi.org/10.1007/s40005-016-0227-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0227-2