Abstract
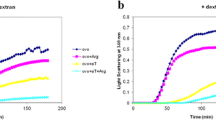
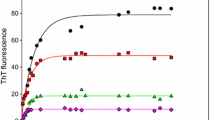
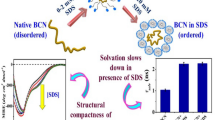
In vitro studies into proteins which are prone to form amyloid are very important. Today, researchers are trying to understand the exact mechanism of amyloid fibril formation because it causes many diseases. κ-Casein is a glycoprotein belonging to the family of milk phosphoprotein which plays an important role in the size, stability and performance of the casein micelles. This study investigates amyloid fibril formation by κ-casein and its prevention by β-casein in the presence and absence of crowding agent, dextran. Interaction between the chaperone and the κ-casein is investigated by thioflavin T fluorescence, intrinsic fluorescence intensity, ANS binding assay and CD spectroscopy. Fluorescence data show that dextran accelerated amyloid fibril formation of κ-casein. β-Casein acts as a molecular chaperone preventing the stress-induced amyloid formation of κ-casein. The effect of β-casein in preventing fibril formation of κ-casein in the presence of dextran was reduced, however, in the absence of dextran. This shows that dextran increases the rate of amyloid formation in κ-casein and causes some structural change in β-casein as assessed by CD spectroscopy. In summary, β-casein interacts with κ-casein and prevents amyloid formation but not as well as it does in the presence of the crowding agent, dextran.





Similar content being viewed by others
References
Asakura S, Oosawa F (1954) On interaction between two bodies immersed in a solution of macromolecules. J Chem Phys 22:1255–1256
Bansal PS, Grieve PA, Marschke RJ, Daly NL, McGhie E, Craik DJ, Alewood PF (2006) Chemical synthesis and structure elucidation of bovine κ-casein. Biochem Biophys Res Commun 340:1098–1103
Cardamone G, Puri NK (1991) 1-Anilino-8-naphthalene sulfonate anion-protein binding depends primarily on ion pair formation. Biochem J 282:589–593
Cheung MS, Klimov D, Thirumalai D (2005) Molecular crowding enhances native state stability and refolding rates. Proc Natl Acad Sci USA 102:4753–4758
Cornelis G, Kruif DE, Huppertz T, Volker S, Andrei U, Petukhov V (2012) Casein micelles and their internal structure. Adv Colloid Interface Sci 172:36–52
Dill KA, Chen HS (1997) From levinthal to pathways to funnels. Nat Struct Biol 4:10–19
Dobson CM (2003) Protein folding and misfolding. Nature 426:884–890
Ecroyd H, Koudelka T, Thorn D, Williams DM, Devlin G, Hoffmann P, Carver JA (2008) Dissociation from the oligomeric state is the rate-limiting step in fibril formation by κ-casein. J Biol Chem 14:9012–9022
Farrell H, Cooke Jr, King P, Hoagland PG, Groves M, Kumosinski T (1996) Particle sizes of casein submicelles and purified κ-casein, comparisons of dynamic light scattering and electron microscopy with predictive three-dimensional molecular models. Am Chem Soc Symp 650:61–79
Fox PF, McSweeney PLH (2003) Milk proteins: general and historical aspects, vol 12, 3rd edn., Advanced dairy chemistry 1: proteinsKluwer Academic, New York, pp 1–48
Fox PF, Sweeney PLH, Swaisgood HE (2003) Chemistry of the caseins. Adv Dairy Chem Part A 139–201
Ghahghaei A, Bathaie SZ, Shahraki A, Asgarabad FR (2011) Comparison of the chaproning action of glycerol and β-casein on aggregation of proteins in the presence of crowding agent. Int J Pept Res Ther 17:101–111
Groves ML, Dower HJ, Farrell HM (1992) Reexamination of the polymeric distributions of κ-casein isolated from bovine milk. J Protein Chem 11:21–28
HadjSadok A, Pitkowski A, Nicolai T, Benyahia L, Moulai-Mostefa N (2008) Characterisation of sodium caseinate as a function of ionic strength, pH and temperature using static and dynamic light scattering. Food Hydrocoll 22:1460–1466
Harrison RS, Sharpe PC, Singh Y, Fairlie DP (2007) Amyloid peptides and proteins in review. Rev Physiol Biochem 10:112–1007
Hatters DM, Minton AP, Howlett GJ (2002) Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J Biol Chem 277:7824–7830
Homouz D, Perham M, Samiotakis A, Cheung MS, Wittung-Stafshede P (2008) Crowded, cell-like environment induces shape changes in aspherical protein. Proc Natl Acad Sci USA 105:11754–11759
Krebs MR, Bromley EH, Donald AM (2005) The binding of thioflavin T to amyloid fibrils localisation and implications. J Struct Biol 149:30–37
Larissa A, Munishkina E, Cooper M, Vladimir N, Uversky, Fink AL (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit 17:1–9
Lazi ML, Veljkovi VB, Vucetict JI, Vrvict MM (1993) Effect of pH and aeration on dextran production by Leuconostoc mesenteroides. Enzyme Microb Technol 15:1684–1958
Leonil J, Henry G, Jouanneau D, Delage M, Forge V, Putaux J-L (2008) Kinetics of fibril formation of bovine κ-casein indicates a conformational rearrangement as a critical step in the process. J Mol Biol 381:1267–1280
Lorenzen PC, Reimerdes EH (1992) Enzymatic dephosphorylation of caseins and creaming behaviour of o/w emulsions stabilised with dephosphorylated casein fractions. Mol Nutr Food Res 6:595–599
Naiki H, Ban T, Hamada D, Hasegawa K, Goto Y (2003) Direct observation of amyloid fibril growth monitored by thioflavin T fluorescence. J Biol Chem 278:16462–16465
Rasmussen LK, Johnsen LB, Tsiora A, Sorensen ES, Thomsen JK, Nielsen NC (1999) Disulphide-linked caseins and casein micelles. Int Dairy J 9:215–218
Roberts CJ (2007) Non-native protein aggregation kinetics. Biotechnol Bioeng 98:927–938
Semisotonov GV, Rodionova NA, Razgulyaev OI, Uvrrsky VN, Gripas AF, Gilmanshin RI (1991) 8-Anilino-1-naphthalene sulfonic acid (ANS) induces folding of acid unfolded cytochrome c to molten globule state as a result of electrostatic interactions. Biopolymers 3:119–128
Shaw MR, Thirumalai D (1991) Free polymer in a colloidal solution. Phys Rev 44:R4797–R4800
Shekar P, Goel S, Rani S, Sarathi D, Alex J, Singh S, Kumar S (2006) κ-Casein deficient mice fail to lactate. Proc Natl Acad Sci USA 103:8000–8005
Snir Y, Kamien RD (2005) Entropically driven helix formation. Science 37:1067
Sreerama N, Woody RW (1999) Molecular dynamics simulations of polypeptide conformations in water: a comparison alpha, beta and poly(Pro)II structures. Proteins Struct Funct Gene 36:400–406
Stagg L, Zhang SQ, Cheung MS, Wittung-Stafshede P (2007) Molecular crowding enhances native structure and stability of alpha/beta protein flavodoxin. Proc Natl Acad Sci USA 104:18976–18981
Stryer LJ (1965) An electrospray ionization mass spectrometry investigation of 1-anilino-8-naphthalene-sulfonate (ANS) binding to proteins. Mol Biol 13:482–495
Swaisgood HE (1992) Chemistry of the caseins In: Advanced dairy chemistry—1: protein; 2nd edn. Elsevier, London, pp 63–110
Thorn DC, Meehan S, Sunde M, Rekas A, Gras SL, MacPhee CE, Dobson CM, Wilson MR, Carver JA (2005) Chemical synthesis and structure elucidation of bovine k-casein (1–44). Biochemistry 44:17027–17036
Turner DC, Brand L (1968) Self-association of 8-anilino-1-naphthalene-sulfonate molecules: spectroscopic characterization and application to the investigation of protein folding. Biochemistry 7:3381–3390
Vladimir N, Anthony L (2006) Protein misfolding, aggregation and conformational diseases. Protein Rev Part A 4:419
West DW (1986) Structure and function of the phosphorylated residues of casein. J Dairy Res 53:333–352
Woody RW, Dunker AK (1996) Aromatic and cystine side-chain circular Dichroism in proteins. In: Circular Dichroism and the conformational analysis of biomolecules, vol 18. Plenum Press, New York, pp 109–157
Zhanga X, Fua X, Zhanga H, Liua C, Jiaoa W, Changa Z (2005) Chaperone-like activity of β-casein. Int J Biochem Cell Biol 37:1232–1240
Zimmerman SB, Minton AP (1993) Macromolecular crowding: biochemical, biophysical, and physiological consequences. Ann Rev Biophys Biomol Struct 22:27–65
Acknowledgments
This article does not contain any studies with human and animal subjects performed by any of the authors. And all authors (A. Ghahghaei and M. M. D. Mianeh) declare that they have no conflict of interest. The authors are thankful to the University of Sistan and Baluchestan for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghahghaei, A., mianeh, M.M.D. Investigating the chaperone activity of β-casein in preventing amyloid formation in κ-casein in the presence of dextran. Journal of Pharmaceutical Investigation 45, 407–413 (2015). https://doi.org/10.1007/s40005-015-0183-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-015-0183-2