Abstract
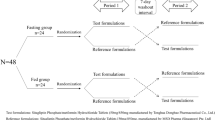
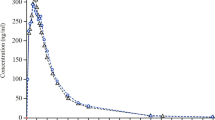
The aim of this study was to evaluate the pharmacokinetics (PK) and bioequivalence (BE) of two metformin tablets. For in vitro evaluation, weight variation, assay and dissolution tests were performed. A randomized, single dose, two-period, cross over study in healthy male fasting volunteers was designed. A 2-week washout period separated the two periods. For analysis of PK parameters blood sampling was performed before and after drug administration in various time points up to 12 h. Metformin concentration in plasma was determined using a developed high performance liquid chromatography method. Both formulations passed the assay, content uniformity, and dissolution tests acceptance value. PK parameters, representing the rate and the extent of metformin absorption were calculated and analyzed for two formulations. The 90 % CI obtained by analysis of variance for the ratios of Cmax, AUC0–t, and AUC0–∞ were 92.14–110.95, 92.72–107.37 and 89.42–110.23 % respectively, meeting the criteria for BE (80–125 %). Administration of a single dose of test and reference formulations did not result in statistically significant differences between in vitro and in vivo BE parameters in healthy Iranian male volunteers. Thus in the case of rate and extent of absorption the test and reference formulations were considered bioequivalent.


Similar content being viewed by others
References
Aburuz S, Millership J, McElnay J (2003) Determination of metformin in plasma using a new ion pair solid phase extraction technique and ion pair liquid chromatography. J Chromatogr B Anal Technol Biomed Life Sci 798(2):203–209
Aburuz S, Millership J, McElnay J (2006) Dried blood spot liquid chromatography assay for therapeutic drug monitoring of metformin. J Chromatogr B Anal Technol Biomed Life Sci 832(2):202–207. doi:10.1016/j.jchromb.2005.12.050
Adikwu MU, Yoshikawa Y, Takada K (2004) Pharmacodynamic-pharmacokinetic profiles of metformin hydrochloride from a mucoadhesive formulation of a polysaccharide with antidiabetic property in streptozotocin-induced diabetic rat models. Biomaterials 25(15):3041–3048. doi:10.1016/j.biomaterials.2003.09.073
Al Hawari S, AlGaai E, Yusuf A, Abdelgaleel A, Hammami MM (2007) Bioequivalence study of two metformin formulations. Arzneimittelforsch 57(4):192–195
Ali J, Arora S, Ahuja A, Babbar AK, Sharma RK, Khar RK, Baboota S (2007) Formulation and development of hydrodynamically balanced system for metformin: in vitro and in vivo evaluation. Eur J Pharm Biopharm 67(1):196–201. doi:10.1016/j.ejpb.2006.12.015
Al-Rimawi F (2009) Development and validation of an analytical method for metformin hydrochloride and its related compound (1-cyanoguanidine) in tablet formulations by HPLC–UV. Talanta 79(5):1368–1371. doi:10.1016/j.talanta.2009.06.004
Andujar-Plata P, Pi-Sunyer X, Laferrere B (2012) Metformin effects revisited. Diabetes Res Clin Pract 95(1):1–9. doi:10.1016/j.diabres.2011.09.022
Batolar LS, Iqbal M, Monif T, Khuroo A, Sharma PL (2012) Bioequivalence and pharmacokinetic comparison of 3 metformin extended/sustained release tablets in healthy Indian male volunteers. Arzneimittelforschung 62(1):22–26. doi:10.1055/s-0031-1295428
Cheng CL, Chou CH (2001) Determination of metformin in human plasma by high-performance liquid chromatography with spectrophotometric detection. J Chromatogr B Biomed Sci Appl 762(1):51–58
Corti G, Cirri M, Maestrelli F, Mennini N, Mura P (2008) Sustained-release matrix tablets of metformin hydrochloride in combination with triacetyl-beta-cyclodextrin. Eur J Pharm Biopharm 68(2):303–309. doi:10.1016/j.ejpb.2007.06.004
D’Avolio A, Sciandra M, Siccardi M, Baietto L, de Requena DG, Bonora S, Di Perri G (2007) A simple and sensitive assay for determining plasma tipranavir concentration in the clinical setting by new HPLC method. J Chromatogr B Anal Technol Biomed Life Sci 848(2):374–378. doi:10.1016/j.jchromb.2006.10.030
Georgita C, Albu F, David V, Medvedovici A (2007) Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI)MS. J Chromatogr B 854(1–2):211–218
Hu LD, Liu Y, Tang X, Zhang Q (2006) Preparation and in vitro/in vivo evaluation of sustained-release metformin hydrochloride pellets. Eur J Pharm Biopharm 64(2):185–192. doi:10.1016/j.ejpb.2006.04.004
Huttunen KM, Rautio J, Leppanen J, Vepsalainen J, Keski-Rahkonen P (2009) Determination of metformin and its prodrugs in human and rat blood by hydrophilic interaction liquid chromatography. J Pharm Biomed Anal 50(3):469–474. doi:10.1016/j.jpba.2009.04.033
ICH Harmonised Tripartite Guideline, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Validation of Analytical Procedure Methodology (1996)
Islambulchilar Z, Valizadeh H, Zakeri-Milani P (2010) Rapid HPLC determination of pioglitazone in human plasma by protein precipitation and its application to pharmacokinetic studies. J AOAC Int 93(3):876–881
Jang SB, Lee YJ, Lim LA, Park KM, Kwon BJ, Woo JS, Kim YI, Park MS, Kim KH, Park K (2010) Pharmacokinetic comparison of controlled-release and immediate-release oral formulations of simvastatin in healthy Korean subjects: a randomized, open-label, parallel-group, single- and multiple-dose study. Clin Ther 32(1):206–216. doi:10.1016/j.clinthera.2010.01.026
Kah Hay Y, Kok Khiang P (1998) Simple high-performance liquid chromatographic method for the determination of metformin in human plasma. J Chromatogr B Biomed Sci Appl 710(1–2):243–246
Kim BH, Shin KH, Kim J, Lim KS, Kim KP, Kim JR, Cho JY, Shin SG, Jang IJ, Yu KS (2009) Pharmacokinetic comparison of a new glimepiride 1-mg + metformin 500-mg combination tablet formulation and a glimepiride 2-mg + metformin 500-mg combination tablet formulation: a single-dose, randomized, open-label, two-period, two-way crossover study in healthy, fasting Korean male volunteers. Clin Ther 31(11):2755–2764. doi:10.1016/j.clinthera.2009.11.001
Lee D, Lim LA, Jang SB, Lee YJ, Chung JY, Choi JR, Kim K, Park JW, Yoon H, Lee J, Park MS, Park K (2011) Pharmacokinetic comparison of sustained- and immediate-release oral formulations of cilostazol in healthy Korean subjects: a randomized, open-label, 3-part, sequential, 2-period, crossover, single-dose, food-effect, and multiple-dose study. Clin Ther 33(12):2038–2053. doi:10.1016/j.clinthera.2011.10.024
Li J, Jin Y, Wang T, Lü X, Li Y (2007) Relative bioavailability and bioequivalence of metforphin hydrochloride extended-released and immediate-released tablets in healthy Chinese volunteers. Eur J Drug Metab Pharm 32(1):21–28. doi:10.1007/bf03190986
Liu Q, Li Z, Shi X, Jiao Z, Zhong M (2009) Simple and sensitive determination of metformin in human plasma using an ion–pair LC method. Chromatographia 70(9):1511–1514. doi:10.1365/s10337-009-1339-x
Marques M, Soares A, Pinto O, Barroso P, Pinto D, Ferreira F, Werneck-Barroso E (2007) Simple and rapid method determination for metformin in human plasma using high performance liquid chromatography tandem mass spectrometry: application to pharmacokinetic studies. J Chromatogr B 852(1/2):308–316
Mistri HN, Jangid AG, Shrivastav PS (2007) Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J Pharm Biomed Anal 45(1):97–106. doi:10.1016/j.jpba.2007.06.003
Najib N, Idkaidek N, Beshtawi M, Bader M, Admour I, Alam SM, Zaman Q, Dham R (2002) Bioequivalence evaluation of two brands of metformin 500 mg tablets (Dialon & Glucophage) in healthy human volunteers. Biopharm Drug Dispos 23(7):301–306. doi:10.1002/bdd.326
Noh YH, Lim HS, Jung JA, Jin SJ, Kim MJ, Kim YH, Park HJ, Bae KS (2012) A single-dose, crossover study comparing the pharmacokinetics and pharmacodynamics of 2 formulations of metformin in healthy volunteers. Int J Clin Pharmacol Ther 50(8):605–613. doi:10.5414/CP201715
Onal A (2009) Spectrophotometric and HPLC determinations of anti-diabetic drugs, rosiglitazone maleate and metformin hydrochloride, in pure form and in pharmaceutical preparations. Eur J Med Chem 44(12):4998–5005. doi:10.1016/j.ejmech.2009.09.003
Porta V, Schramm S, Kano EK, Koono EE, Armando YP, Fukuda K, Serra CH (2008) HPLC–UV determination of metformin in human plasma for application in pharmacokinetics and bioequivalence studies. J Pharm Biomed Anal 46(1):143–147. doi:10.1016/j.jpba.2007.10.007
Shin KH, Kim SE, Yoon SH, Cho JY, Jang IJ, Shin SG, Yu KS (2011) Pharmacokinetic comparison of a new sustained-release formulation of glimepiride/metformin 1/500 mg combination tablet and a sustained-release formulation of glimepiride/metformin 2/500 mg combination tablet in healthy Korean male volunteers: a randomized, 2-sequence, 2-period, 2-treatment crossover study. Clin Ther 33(11):1809–1818. doi:10.1016/j.clinthera.2011.10.003
Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Investig 117(5):1422–1431. doi:10.1172/JCI30558
Stepensky D, Friedman M, Srour W, Raz I, Hoffman A (2001) Preclinical evaluation of pharmacokinetic-pharmacodynamic rationale for oral CR metformin formulation. J Control Release 71(1):107–115
Tache F, David V, Farca A, Medvedovici A (2001) HPLC–DAD determination of metformin in human plasma using derivatization with p-nitrobenzoyl chloride in a biphasic system. Microchem J 68(1):13–19
Valizadeh H, Zakeri-Milani P, Islambulchilar Z, Tajerzadeh H (2006) A simple and rapid high-performance liquid chromatography method for determining furosemide, hydrochlorothiazide, and phenol red: applicability to intestinal permeability studies. J AOAC Int 89(1):88–93
Valizadeh H, Barghi L, Jalilian H, Islambulchilar Z, Zakeri-Milani P (2009) Bioequivalence of fexofenadine tablet formulations assessed in healthy Iranian volunteers. ArzneimittelForsch 59(7):345–349
Valizadeh H, Nemati M, Hallaj-Nezhadi S, Ansarin M, Zakeri-Milani P (2010) Single dose bioequivalence study of alpha-methyldopa tablet formulations using a modified HPLC method. Arzneimittelforsch 60(10):607–611
Vlahov V, Thyroff-Friesinger U, Koytchev R, Bakracheva N, Gatchev E (2005) Bioequivalence studies with metformin: comparability of reference tablets from different origins. Int J Clin Pharmacol Ther 43(9):457–462
Yu B, Pugazhenthi S, Khandelwal RL (1994) Effects of metformin on glucose and glucagon regulated gluconeogenesis in cultured normal and diabetic hepatocytes. Biochem Pharmacol 48(5):949–954. doi:0006-2952(94)90365-4
Yuen KH, Wong JW, Billa N, Julianto T, Toh WT (1999) Bioequivalence of a generic metformin tablet preparation. Int J Clin Pharmacol Ther 37(7):319–322
Zakeri-Milani P, Barzegar-Jalali M, Tajerzadeh H, Azarmi Y, Valizadeh H (2005) Simultaneous determination of naproxen, ketoprofen and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal 39(3–4):624–630. doi:10.1016/j.jpba.2005.04.008
Zakeri-Milani P, Valizadeh H, Islambulchilar Z (2008) Comparative bioavailability study of two cefixime formulations administered orally in healthy male volunteers. ArzneimittelForsch 58(2):97–100
Zakeri-Milani P, Valizadeh H, Ghanbarzadeh S, Nemati M (2009) Pharmacokinetics and comparative bioavailability study of two clarithromycin suspensions following administration of a single oral dose to healthy volunteers. ArzneimittelForsch 59(8):429–432
Zakeri-Milani P, Valizadeh H, Islambulchilar Z, Nemati M (2010) Pharmacokinetic and bioequivalence study of two brands of valsartan tablets in healthy male volunteers. ArzneimittelForsch 60(2):76–80
Zhang Q, Tao Y, Zhu Y, Zhu D (2010) Bioequivalence and pharmacokinetic comparison of two mycophenolate mofetil formulations in healthy Chinese male volunteers: an open-label, randomized-sequence, single-dose, two-way crossover study. Clin Ther 32(1):171–178. doi:10.1016/j.clinthera.2010.01.013
Acknowledgments
The authors would like to thank the authorities of the Faculty of Pharmacy, Tabriz University of Medical Sciences for providing analytical facilities and Exir Pharmaceutical Company (Iran) for financial support. This paper is based on a Pharm D thesis (Number 3574) submitted in Faculty of Pharmacy, Tabriz University of Medical Sciences.
Conflict of interest
The authors report no conflict of interest in the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valizadeh, H., Nayyeri-Maleki, P., Ghanbarzadeh, S. et al. Pharmacokinetics and bioequivalence of two brands of metformin 500 mg tablets in Iranian healthy volunteers. Journal of Pharmaceutical Investigation 44, 61–68 (2014). https://doi.org/10.1007/s40005-013-0102-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-013-0102-3