Abstract
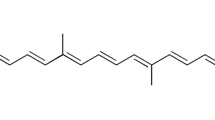
Topical formulation of retinyl retinoate (RR) was developed with nanostructured lipid carriers (NLCs), composed of Compritol or Precirol as a solid lipid, canola oil as an oil, and Tween 80 as a surfactant. Hot high pressure homogenization method was efficiently employed to yield a homogenous nanodispersion in the size range of 230–300 nm with PDI values of <0.2, regardless of lipid selection. Precirol-based NLC (P-NLC) showed higher drug entrapment than that of Compritol-based NLC (C-NLC): RR encapsulation efficiency (%) of P- and C-NLC was 97.8 and 93.8 in average, respectively; drug loading (mg RR/g lipid) was 89.6 and 83.3 in average, respectively. Processing condition at relatively low temperature of 60 °C was a key factor for maintaining RR stability. Drug release of P-NLC was greater than that of C-NLC, even though both NLCs revealed controlled release pattern. Therefore, P-NLC system was considered as a suitable vehicle for topical drug delivery, especially for heat-labile ingredient like RR.





Similar content being viewed by others
References
Choi WS, Cho HI, Lee HY, Lee SH, Choi YW (2010) Enhanced occlusiveness of nanostructured lipid carrier (NLC)-based carbogel as a skin moisturizing vehicle. J Pharm Invest 40:373–378
de Lorgeril M, Salen P (2006) The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr 9:118–123
de Vringer T (1999) Topical preparation containing a suspension of solid lipid particles. US Patent No. 5904932
Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, Tavakkol A, Yi JY, Griffiths CE, Elder JT (1995) Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol 105:549–556
Kim BH, Lee YS, Kang KS (2003) The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett 146:65–73
Kim HJ, Kim BR, Kim H, Um SJ, Lee JD, Ryoo HC, Jung HI (2008) Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg Med Chem 16:6387–6393
Kim H, Kim N, Jung S, Mun J, Kim J, Kim B, Lee J, Ryoo H, Jung H (2010) Improvement in skin wrinkles from the use of photostable retinyl retinoate: a randomized controlled trial. Br J Dermatol 162:497–502
Kristl J, Volk B, Gasperlin M, Sentjurc M, Jurkovic P (2003) Effect of colloidal carriers on ascorbyl palmitate stability. Eur J Pharm Sci 19:181–189
Kuwahara H, Kanazawa A, Wakamatu D, Morimura S, Kida K, Akaike T, Maeda H (2004) Antioxidative and antimutagenic activities of 4-vinyl-2,6-dimethoxyphenol (Canolol) isolated from canola oil. J Agric Food Chem 52:4280–4287
Lim SJ, Kim CK (2002) Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int J Pharm 243:135–146
Liu J, Hu W, Chen H, Ni Q, Xu H, Yang J (2007) Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm 328:191–195
Morais J, Santos O, Delicato T, Goncalves R, Rocha-Filho P (2006) Physicochemical characterization of canola oil/water nano-emulsions obtained by determination of required HLB number and emulsion phase inversion. J Disper Sci Technol 27:109–115
Müller RH, Radtke M, Wissing SA (2002) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:131–155
Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC (1997) Current use and future potential role of retinoids in dermatology. Drugs 53:358–388
Pardeike J, Schwabe K, Müller RH (2010) Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect. Int J Pharm 396:166–173
Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singha M (2010) Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release 144:233–241
Puglia C, Blasi P, Rizza L, Schoubben A, Bonina F, Rossi C, Ricci M (2008) Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm 357:295–304
Roos TC, Jugert FK, Merk HF, Bickers DR (1998) Retinoid Metabolism in the skin. Pharmacol Rev 50:315–333
Schurer NY, Elias PM (1991) The biochemistry and function of stratum corneum lipids. Adv Lipid Res 24:27–56
Shen H, Zhong M (2006) Preparation and evaluation of self microemulsifying drug delivery system (SMEDDs) containing atorvastatin. J Pharm Pharmacol 58:1183–1191
Teeranachaideekul V, Müller RH, Junvaprasert VB (2007) Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—effects of formulation parameters on physicochemical stability. Int J Pharm 340:198–206
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M (2000) Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65:403–418
Wanasundara U, Amarowicz R, Shahidi F (1994) Isolation and identification of an antioxidative component in canola meal. J Agric Food Chem 42:1285–1290
Wissing SA, Müller RH (2003) Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm 254:65–68
Wissing S, Lippacher A, Müller RH (2001) Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J Cosmet Sci 52:313–324
Zhai H, Maibach HI (2001) Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis 44:201–206
Acknowledgments
This study was supported by Seoul R & BD program (SS100001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S.G., Jeong, J.H., Kim, S.R. et al. Topical formulation of retinyl retinoate employing nanostructured lipid carriers. Journal of Pharmaceutical Investigation 42, 243–250 (2012). https://doi.org/10.1007/s40005-012-0036-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-012-0036-1