Abstract
Background
This meta-analysis was conducted to compare the efficacy of ceftazidime-avibactam combination therapy with that of monotherapy in the treatment of carbapenem-resistant Gram-negative bacterial (CR-GNB).
Methods
A literature search of PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov was conducted until September 1, 2023. Only studies that compared CZA combination therapy with monotherapy for CR-GNB infections were included.
Results
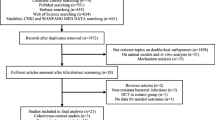
A total of 25 studies (23 retrospective observational studies and 2 prospective studies) involving 2676 patients were included. There was no significant difference in 30-day mortality between the study group receiving combination therapy and the control group receiving monotherapy (risk ratio [RR] 0.91; 95% confidence interval [CI] 0.71–1.18). In addition, no significant differences were observed between the study and the control group in terms of in-hospital mortality (RR 1.00; 95% CI 0.79–1.27), 14-day mortality (RR 1.54; 95% CI 0.24–9.91), 90-day mortality (RR 1.18; 95% CI 0.62–2.22), and clinical cure rate (RR 0.95; 95% CI 0.84–1.08). However, the combination group had a borderline higher microbiological eradication rate than the control group (RR 1.15; 95% CI 1.00–1.32).
Conclusions
Compared to monotherapy, CZA combination therapy did not yield additional clinical benefits. However, combination therapy may be associated with favorable microbiological outcomes.






Similar content being viewed by others
Data availability
Our study is a Systematic Review/Meta-analysis. The datasets analyzed during the current study are available in the published pooled study. Also, the datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399:629–55.
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–27.
Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis. 2018;5:ofy150.
Kempf M, Arhin FF, Stone G, Utt E. Ceftazidime-avibactam activity against Gram-negative respiratory isolates collected between 2018 and 2019. J Glob Antimicrob Resist. 2022;31:239–47.
La Bella G, Lopizzo T, Lupo L, Angarano R, Curci A, Manti B, et al. In vitro activity of ceftazidime/avibactam against carbapenem-nonsusceptible Klebsiella penumoniae isolates collected during the first wave of the SARS-CoV-2 pandemic: a Southern Italy, multicenter, surveillance study. J Glob Antimicrob Resist. 2022;31:236–8.
Tsai CH, Lee NY, Chao CM, Chen CC, Lai CC, Ho CH, et al. Emergence and dissemination of multidrug-resistant escherichia coli ST8346 coharboring bla(NDM-5) and bla(OXA-181) in Southern Taiwan, 2017–2021. J Infect Public Health. 2023;16:1675–81.
Wise MG, Karlowsky JA, Lemos-Luengas EV, Valdez RR, Sahm DF. Epidemiology and in vitro activity of ceftazidime-avibactam and comparator agents against multidrug-resistant isolates of enterobacterales and Pseudomonas aeruginosa collected in Latin America as part of the atlas surveillance program in 2015–2020. Braz J Infect Dis. 2023;27: 102759.
Wise MG, Karlowsky JA, Mohamed N, Kamat S, Sahm DF. In vitro activity of aztreonam-avibactam against enterobacterales isolates collected in Latin America, Africa/Middle East, Asia, and eurasia for the atlas global surveillance program in 2019–2021. Eur J Clin Microbiol Infect Dis. 2023;42:1135–43.
Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, Wardman A, et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis. 2016;16:661–73.
Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind Phase II trial. J Antimicrob Chemother. 2013;68:1183–92.
Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, et al. Efficacy and safety of ceftazidime-avibactam Plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis. 2016;62:1380–9.
Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, et al. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49:579–88.
Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18:285–95.
Vazquez JA, González Patzán LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28:1921–31.
Wagenlehner FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: recapture, a phase 3 randomized trial program. Clin Infect Dis. 2016;63:754–62.
Gaibani P, Lewis RE, Volpe SL, Giannella M, Campoli C, Landini MP, et al. In vitro interaction of ceftazidime-avibactam in combination with different antimicrobials against KPC-producing Klebsiella pneumoniae clinical isolates. Int J Infect Dis. 2017;65:1–3.
Mataracı Kara E, Yılmaz M, Özbek ÇB. In vitro activities of ceftazidime/avibactam alone or in combination with antibiotics against multidrug-resistant Acinetobacter baumannii isolates. J Glob Antimicrob Resist. 2019;17:137–41.
Gaudereto JJ, PerdigãoNeto LV, Leite GC, RuedasMartins R, do BoasPrado GV, Rossi F, et al. Synergistic effect of ceftazidime-avibactam with meropenem against panresistant, carbapenemase-harboring acinetobacter baumannii and serratia marcescens investigated using time-kill and disk approximation assays. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/AAC.02367-18.
Kroemer N, Martens M, Decousser JW, Grégoire N, Nordmann P, Wicha SG. Evaluation of in vitro pharmacodynamic drug interactions of ceftazidime/avibactam and fosfomycin in escherichia coli. J Antimicrob Chemother. 2023;78:2524–34.
Romina PE, Lucía A, Leticia C, Federica F, Pablo Á, Verónica S, et al. In vitro effectiveness of ceftazidime-avibactam in combination with aztreonam on carbapenemase-producing Enterobacterales. J Glob Antimicrob Resist. 2023;35:62–6.
Boattini M, Bianco G, Charrier L, Comini S, Iannaccone M, Almeida A, et al. Rapid diagnostics and ceftazidime/avibactam for KPC-producing Klebsiella pneumoniae bloodstream infections: impact on mortality and role of combination therapy. Eur J Clin Microbiol Infect Dis. 2023;42:431–9.
Alqahtani H, Alghamdi A, Alobaidallah N, Alfayez A, Almousa R, Albagli R, et al. Evaluation of ceftazidime/avibactam for treatment of carbapenemase-producing carbapenem-resistant Enterobacterales with OXA-48 and/or NDM genes with or without combination therapy. JAC Antimicrob Resist. 2022;4:dlac104.
Lin J, Zhang L, Zhou M, Tian X, Chen J, Lu M, et al. Combination therapy of ceftazidime/avibactam for the treatment of patients infected with carbapenem-resistant klebsiella pneumoniae: a multicenter retrospective study. Infect Dis Ther. 2023;12:2165–77.
King M, Heil E, Kuriakose S, Bias T, Huang V, El-Beyrouty C, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61:10–128.
Sousa A, Pérez-Rodríguez MT, Soto A, Rodríguez L, Pérez-Landeiro A, Martínez-Lamas L, et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing enterobacteriaceae. J Antimicrob Chemother. 2018;73:3170–5.
Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, et al. Efficacy of ceftazidime-avibactam salvage therapy in Patients with infections caused by klebsiella pneumoniae carbapenemase-producing k pneumoniae. Clin Infect Dis. 2019;68:355–64.
Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, Venditti M, et al. Ceftazidime-avibactam use for klebsiella pneumoniae carbapenemase-producing k pneumoniae Infections: a retrospective observational multicenter study. Clin Infect Dis. 2021;73:1664–76.
Li D, Fei F, Yu H, Huang X, Long S, Zhou H, et al. ceftazidime-avibactam therapy versus ceftazidime-avibactam-based combination therapy in patients with carbapenem-resistant gram-negative pathogens: a meta-analysis. Front Pharmacol. 2021;12: 707499.
Fiore M, Alfieri A, Di Franco S, Pace MC, Simeon V, Ingoglia G, et al. Ceftazidime-avibactam combination therapy compared to ceftazidime-avibactam monotherapy for the treatment of severe infections due to carbapenem-resistant Pathogens: a systematic review and network meta-analysis. Antibiotics (Basel). 2020;9:388.
Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents. 2019;54:735–40.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.
Jonathan ACS, Miguel AH, Barnaby CR, Jelena S, Nancy DB, Meera V, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis Dio. 2023. https://doi.org/10.1093/cid/ciad428.
Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among Patients with carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62:10–128.
Nagvekar V, Shah A, Unadkat VP, Chavan A, Kohli R, Hodgar S, et al. Clinical outcome of patients on ceftazidime-avibactam and combination therapy in carbapenem-resistant enterobacteriaceae. Indian J Crit Care Med. 2021;25:780–4.
Davido B, Crémieux AC, Vaugier I, De Truchis P, Hamami K, Laurent F, et al. Efficacy of ceftazidime/avibactam in various combinations for the treatment of experimental osteomyelitis in rabbits caused by OXA-48-/ESBL-producing escherichia coli. J Antimicrob Chemother. 2023;78:1211–8.
Mantzana P, Protonotariou E, Kassomenaki A, Meletis G, Tychala A, Keskilidou E, et al. In Vitro synergistic activity of antimicrobial combinations against carbapenem- and colistin-resistant acinetobacter baumannii and klebsiella pneumoniae. Antibiotics (Basel). 2023;12:93.
Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, Chen L, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-Avibactam resistance among patients treated for carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis. 2016;63:1615–8.
Both A, Büttner H, Huang J, Perbandt M, Belmar Campos C, Christner M, et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR klebsiella pneumoniae isolate. J Antimicrob Chemother. 2017;72:2483–8.
Mishuk AU, Strich J, Warner S, Sun J, Malik S, Lawandi A, et al. 652. Ceftazidime-avibactam alone or as combination therapy? Multicenter retrospective cohort analysis of clinical outcomes in patients with carbapenem-resistant Gram-negative Infection. Open Forum Infect Dis. 2022. https://doi.org/10.1093/ofid/ofac492.704.
Zhang F, Zhong J, Ding H, Liao G. Efficacy of ceftazidime-avibactam in the treatment of carbapenem-resistant klebsiella pneumoniae infection after kidney transplantation. Infect Drug Resist. 2021;14:5165–74.
Kuang H, Zhong C, Wang Y, Ye H, Ao K, Zong Z, et al. Clinical characteristics and outcomes of patients with multidrug-resistant gram-negative bacterial infections treated with ceftazidime/avibactam. J Glob Antimicrob Resist. 2020;23:404–7.
De la Calle C, Rodríguez O, Morata L, Marco F, Cardozo C, García-Vidal C, et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents. 2019;53:520–4.
Jorgensen SCJ, Trinh TD, Zasowski EJ, Lagnf AM, Bhatia S, Melvin SM, et al. Evaluation of the INCREMENT-CPE, pitt bacteremia and qPitt scores in patients with carbapenem-resistant enterobacteriaceae infections treated with ceftazidime-avibactam. Infect Dis Ther. 2020;9:291–304.
Castón JJ, Gallo M, García M, Cano A, Escribano A, Machuca I, et al. Ceftazidime-avibactam in the treatment of infections caused by KPC-producing klebsiella pneumoniae: factors associated with clinical efficacy in a single-center cohort. Int J Antimicrob Agents. 2020;56: 106075.
Ackley R, Roshdy D, Meredith J, Minor S, Anderson WE, Capraro GA, et al. Meropenem-vaborbactam versus ceftazidime-avibactam for treatment of carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother. 2020;64:10–128.
Chen L, Han X, Li Y, Li M. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant enterobacterales. Antimicrob Agents Chemother. 2021;65: e0069821.
Zheng G, Zhang J, Wang B, Cai J, Wang L, Hou K, et al. Ceftazidime-avibactam in combination with In vitro Non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther. 2021;10:1699–713.
Gu J, Xu J, Zuo TT, Chen YB. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant klebsiella pneumoniae: ceftazidime-avibactam against CR-KP infections. J Glob Antimicrob Resist. 2021;26:20–5.
Rathish B, Wilson A, Warrier A, Prakash S, Babu R, Joy S. Clinical outcomes in carbapenem-resistant enterobacteriaceae infections treated with ceftazidime-avibactam: a single-center observational study. Cureus. 2021;13: e13081.
Zheng G, Cai J, Zhang L, Chen D, Wang L, Qiu Y, et al. Ceftazidime/avibactam-based versus polymyxin B-based therapeutic regimens for the treatment of carbapenem-resistant klebsiella pneumoniae infection in critically Ill patients: a retrospective cohort study. Infect Dis Ther. 2022;11:1917–34.
Luterbach CL, Qiu H, Hanafin PO, Sharma R, Piscitelli J, Lin FC, et al. A systems-based analysis of mono- and combination therapy for carbapenem-resistant klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2022;66: e0059122.
Zhuang HH, Chen Y, Hu Q, Long WM, Wu XL, Wang Q, et al. Efficacy and mortality of ceftazidime/avibactam-based regimens in carbapenem-resistant Gram-negative bacteria infections: a retrospective multicenter observational study. J Infect Public Health. 2023;16:938–47.
Pérez-Nadales E, Fernández-Ruiz M, Natera AM, Gutiérrez-Gutiérrez B, Mularoni A, Russelli G, et al. Efficacy of ceftazidime-avibactam in solid organ transplant recipients with bloodstream infections caused by carbapenemase-producing klebsiella pneumoniae. Am J Transplant. 2023;23:1022–34.
Zhen S, Zhao Y, Chen Z, Zhang T, Wang J, Jiang E, et al. Assessment of mortality-related risk factors and effective antimicrobial regimens for treatment of bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa in patients with hematological diseases. Front Cell Infect Microbiol. 2023;13:1156651.
Author information
Authors and Affiliations
Contributions
WH, HYL and HJT: conception or design of the work. WH, MHC, WWT, and CCL: analysis, WH and CCL: interpretation of data for the work; WH and HYL: Drafting the work. HYL and HJT: Reviewing it critically for important intellectual content. All authors had final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there was no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsu, W., Chuang, MH., Tsai, WW. et al. Ceftazidime-avibactam combination therapy versus monotherapy for treating carbapenem-resistant gram-negative infection: a systemic review and meta-analysis. Infection (2024). https://doi.org/10.1007/s15010-024-02277-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02277-y