Abstract
Purpose
Major bleedings have been described with cefazolin. The objective was to determine the frequency of bleeding events in cefazolin-treated patients and to identify risk factors for these complications.
Methods
Monocenter prospective observational study of all consecutive cefazolin-treated patients. Patients benefited from a daily clinical assessment of bleedings and a twice-a-week blood sampling including hemostasis. Bleedings were classified according to the International Society on Thrombosis and Hemostasis classification: major, clinically relevant non-major bleedings (CRNMB) and minor bleedings.
Results
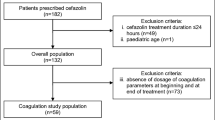
From September 2019 to July 2020, 120 patients were included, with a mean age of 59.4 (± 20.7) years; 70% of them (84/120) were men. At least 1 CRNMB or major bleeding were observed in 10% of the patients (12/120). Compared to patients with no or minor bleeding, patients with CRNMB or major bleeding were, upon start of cefazolin, more frequently hospitalized in an intensive care unit (7/12, 58.3%, vs. 12/108, 11.1%, P < 0.001, respectively) and receiving vitamin K antagonists (4/12, 33.3%, vs. 8/108, 7.4%, P = 0.019, respectively). After multivariate analysis, patients receiving vitamin K antagonists the day prior bleeding and/or treated for endocarditis were factors associated with an increased risk of CRNMB or major bleeding (odd ratio 1.36, confidence interval 95%, 1.06–1.76, P = 0.020 and 1.30, 1.06–1.61, P = 0.015, respectively).
Conclusions
Bleeding events associated with cefazolin treatment are frequent. Close clinical monitoring should be performed for patients treated for endocarditis and/or receiving vitamin K antagonists. Hemostasis work-up could be restricted to these patients.

Similar content being viewed by others
Data availability
Data are available upon reasonable request to the authors.
References
Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect. 2013;14:73–156.
Wu C-K, Wang J-H, Lee C-H, et al. The outcome of prophylactic intravenous cefazolin and ceftriaxone in cirrhotic patients at different clinical stages of disease after endoscopic interventions for acute variceal hemorrhage. PLoS ONE. 2013;8:e61666.
Alotaibi AF, Mekary RA, Zaidi HA, Smith TR, Pandya A. Safety and efficacy of antibacterial prophylaxis after craniotomy: a decision model analysis. World Neurosurg. 2017;105:906–12.e5.
Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–86.
Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128.
Shi C, Xiao Y, Zhang Q, et al. Efficacy and safety of cefazolin versus antistaphylococcal penicillins for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia: a systematic review and meta-analysis. BMC Infect Dis. 2018;18:508.
Youngster I, Shenoy ES, Hooper DC, Nelson SB. Comparative evaluation of the tolerability of cefazolin and nafcillin for treatment of methicillin-susceptible Staphylococcus aureus infections in the outpatient setting. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59:369–75.
Li J, Echevarria KL, Hughes DW, Cadena JA, Bowling JE, Lewis JS. Comparison of cefazolin versus oxacillin for treatment of complicated bacteremia caused by methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 2014;58:5117–24.
Burdet C, Loubet P, Le Moing V, et al. Efficacy of cloxacillin versus cefazolin for methicillin-susceptible Staphylococcus aureus bacteraemia (CloCeBa): study protocol for a randomised, controlled, non-inferiority trial. BMJ Open. 2018;8:e023151.
Shimada K, Matsuda T, Inamatsu T, Urayama K. Bleeding secondary to vitamin K deficiency in patients receiving parenteral cephem antibiotics. J Antimicrob Chemother. 1984;14(Suppl B):325–330.
Barnes T, Yan S, Kaakeh Y. Necrotizing esophagitis and bleeding associated with cefazolin. Ann Pharmacother. 2014;48:1214–8.
Gay E, Barthel A, Rouzic N, et al. Cefazolin and coagulation disorders: a case report. Ann Biol Clin (Paris). 2018;76:104–6.
Angles E, Mouton C, Perino J, Remy A, Ouattara A. Hypoprothrombinemia and severe perioperative haemorrhagic complications in cardiac surgery patients treated with high-dose cefazolin for infective endocarditis. Anaesth Crit Care Pain Med. 2018;37:167–70.
Résumé des caractéristiques du produit - CEFAZOLINE VIATRIS 1 g, poudre pour solution injectable (IM-IV) - Base de données publique des médicaments. https://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=61242331&typedoc=R. Accessed 28 Nov 2022.
Shearer MJ, Bechtold H, Andrassy K, et al. Mechanism of cephalosporin-induced hypoprothrombinemia: relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J Clin Pharmacol. 1988;28:88–95.
Wood TC, Johnson KL, Naylor S, Weinshilboum RM. Cefazolin administration and 2-methyl-1,3,4-thiadiazole-5-thiol in human tissue: possible relationship to hypoprothrombinemia. Drug Metab Dispos Biol Fate Chem. 2002;30:1123–8.
Strazzulla A, Chakvetadze C, Picque M, et al. Evolution of haemostatic parameters and risk of bleeding during treatment with cefazolin. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2019;38:177–83.
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Daniel W. Biostatistics: a foundation for analysis in the health sciences. New York: Wiley; 1999.
Guilhaumou R, Benaboud S, Bennis Y, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care Lond Engl. 2019;23:104.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4.
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies—PubMed. https://pubmed.ncbi.nlm.nih.gov/18064739/. Accessed 23 Nov 2022.
Rao SN, Rhodes NJ, Lee BJ, et al. Treatment outcomes with cefazolin versus oxacillin for deep-seated methicillin-susceptible Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother. 2015;59:5232–8.
Twilla JD, Algrim A, Adams EH, Samarin M, Cummings C, Finch CK. Comparison of nafcillin and cefazolin for the treatment of methicillin-susceptible Staphylococcus aureus bacteremia. Am J Med Sci. 2020;360:35–41.
Chien T-L, Hsiao F-Y, Chen L-J, Wen Y-W, Lin S-W. Development and validation of a risk scoring system for cephamycin-associated hemorrhagic events. Sci Rep. 2019;9:12905.
Wang W, Liu Y, Yu C, et al. Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: a retrospective cohort study. Expert Opin Drug Saf. 2020;19:339–47.
Raine J, Spooner A. Minutes from the meeting on 30 August–02 September 2016. 2016.
Abbas S, Ihle P, Harder S, Schubert I. Risk of bleeding and antibiotic use in patients receiving continuous phenprocoumon therapy. A case-control study nested in a large insurance- and population-based German cohort. Thromb Haemost. 2014;111:912–922.
Siguret V, Pautas E, Gouin-Thibault I. Warfarin therapy: influence of pharmacogenetic and environmental factors on the anticoagulant response to warfarin. Vitam Horm. 2008;78:247–64.
Acknowledgements
The results of this study were presented as a poster at the Journées nationales d’infectiologie, the French Infectious Disease Society congress in 2021. The authors would like to thank Bastien Rance and Estelle Lu for the extraction of the biological data from the database of the Assistance Publique-Hôpitaux de Paris. The authors would like to thank Marion Lacasse, Marie Berleur, Ségolène Gendraux, Déborah Porez, Pauline Martinet and Matthieu Petit for the help during data collection.
Funding
Statistical analyses were performed using a grant from AP-HP (Fonds APRES “Appui aux Projets pour le REnforcement du Sens, 2020, Assistance-Publique Hôpitaux de Paris).
Author information
Authors and Affiliations
Contributions
EG and DL contributed to the conceptualization of the protocol, the investigation, the interpretation of the statistical analysis and wrote (original draft) the manuscript. NG participated in the investigation, the interpretation of the statistical analysis and writing (reviewing and editing) of the final manuscript. YT performed the formal analyses, participated in their interpretation and wrote (reviewing and editing) the final manuscript. ML and BK participated in the investigation and writing (reviewing and editing) of the final manuscript. DS, BS, TC, ML, EB, ALL contributed to the conceptualization of the protocol and wrote (reviewing and editing) the final manuscript. All authors gave their final approval for the version of the manuscript to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not declare any conflict of interests.
Ethical approval
The study was approved by a national expert committee (reference 2019–06–01) and was declared to the CNIL (Comité national de l’informatique et des libertés, reference 2213058 v 0).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gras, E., Tran, Y., Kably, B. et al. Prospective assessment of the frequency of and risk factors for bleeding events in patients treated with cefazolin. Infection 52, 557–566 (2024). https://doi.org/10.1007/s15010-023-02145-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02145-1