Abstract
Purpose
Overall, insertion of central venous catheter (CVC) into femoral veins (FV) has been shown to be associated with a higher risk of infection compared with subclavian and internal jugular (IJV/SCV) CVC, but no data are available on the impact of the FV insertion site on the CVC-related bloodstream infections (CRBSI) risk in patients with cancer. The objective of the study is to compare CRBSI rates and incidences of FV with those of internal jugular and subclavian vein (IJV/SCV CVC) as observed in the prospective SECRECY registry.
Methods
SECRECY is an ongoing observational, prospective, clinical CRBSI registry active in six departments of hematology/oncology in Germany. Each case of FV CVC was matched at a ratio of 1:1 to a case with IJV/SCV CVC. The propensity score was estimated using a multivariable logistic regression model adjusting for age, sex, cancer type, and duration of indwelling catheter.
Results
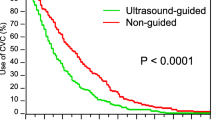
Of 4268 CVCs included in this analysis, 52 (1.2%) were inserted into the FV and 4216 (98.8%) into the IJV/SCV. 52 cases of FV CVC were matched with 52 IJV/SCV CVC. There was no significant difference in the CRBSI rate (3.8% vs. 9.6%), the CRBSI incidence (5.7 vs. 14.2/1000 CVC days), and the median CVC time (5.5 vs. 5 days) between the FV and the IJV/SCV group.
Conclusion
Based on this data, inserting FV CVCs in patients with cancer does, at least in the short-term, not appear to be associated with an increased risk of CRBSI as compared to IJV/SCV CVC.

Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Böll B, Schalk E, Buchheidt D, et al. Central venous catheter-related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann Hematol. 2021;100:239–59.
O’Grady NP, Alexander M, Burns LA, et al. Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162-93.
Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373:1220–9.
Arvaniti K, Lathyris D, Blot S, et al. Cumulative evidence of randomized controlled and observational studies on catheter related infection risk of central venous catheter insertion site in ICU patients: a pairwise and network meta-analysis. Crit Care Med. 2017;45:e437–48.
Parienti JJ. Catheter-related bloodstream infection in jugular versus subclavian central catheterization. Crit Care Med. 2017;45:e734–5.
Rixecker T, Lesan V, Ahlgrimm M, et al. Insertion site of central venous catheter correlates with catheter-related infectious events in patients undergoing intensive chemotherapy. Bone Marrow Transplant. 2021;56:195–201.
Heidenreich D, Hansen E, Kreil S, et al. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am J Hematol. 2022;97:303–10.
Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Prävention von Infektionen, die von Gefäßkathetern ausgehen Teil 1 – Nichtgetunnelte zentralvenöse Katheter. Bundesgesundheitsbl. 2017;60:171–206.
Hentrich M, Schalk E, Schmidt-Hieber M, et al. Central venous catheter-related infections in hematology and oncology: 2012 updated guidelines on diagnosis, management and prevention by the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology. Ann Oncol. 2014;25:936–47.
Schalk E, Tölle D, Schulz S, et al. Identifying haematological cancer patients with high risk for central venous catheter (CVC)-related bloodstream infections at the time point of CVC insertion. 29th European Congress of Clinical Microbiology and Infectious Diseases, April 13–16, 2019, Amsterdam, The Netherlands, abstract P2556. https://www.escmid.org/escmid_publications/escmid_elibrary. Published 2019. Accessed 30 May 2022.
Ho DE, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. https://doi.org/10.18637/jss.v042.i08.
Timsit JF, Bouadma L, Mimoz O, et al. Jugular versus femoral short-term catheterization and risk of infection in intensive care unit patients. Causal analysis of two randomized trials. Am J Respir Crit Care Med. 2013;188:1232–9.
Heidenreich D, Hansen E, Kreil S, et al. Influence of the insertion site on central venous catheter-related complications in patients undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26:1189–94.
Snarski E, Stringer J, Mikulska M, et al. Risk of infectious complications in adult patients after allogeneic hematopoietic stem cell transplantation depending on the site of central venous catheter insertion-multicenter prospective observational study, from the IDWP EBMT and Nurses Group of EBMT. Bone Marrow Transplant. 2021;56:2929–33.
Acknowledgements
The authors thank all SECRECY staff for their great support.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
MH was involved in patient management, designed the study, collected and interpreted the data, and wrote the manuscript. BB was involved in patient management, performed statistical analyses, and revised the manuscript. DT was involved in patient management, and revised the manuscript. JP was involved in patient management, and revised the manuscript. TS was involved in patient management, collected the data, and revised the manuscript. J-HN was involved in patient management, collected the data, and revised the manuscript. MS-H was involved in patient management, and revised the manuscript. JN was involved in patient management, collected the data, and revised the manuscript. EF was involved in patient management, collected the data, and revised the manuscript. ES was involved in patient management, designed the study, collected and analyzed the data, and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The registry was approved by the central ethics committee (Magdeburg University Hospital, approval no. 84/14) as well as by respective local ethic committees.
Patient consent to participate
Given the nature of routine clinical data and the anonymization of patient data, written informed consent was not required within the study.
Patient consent to publish
Written informed consent was not required within the study.
Supplementary Information
Below is the link to the electronic supplementary material.
15010_2023_2029_MOESM2_ESM.tiff
Supplementary Figure 1. Cumulative CRBSI incidence (propensity score matching with 52 FV and 52 IJV CVC; P = .269 [log-rank test]). Supplementary file2 (TIFF 9229 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hentrich, M., Böll, B., Teschner, D. et al. Impact of the insertion site of central venous catheters on central venous catheter-related bloodstream infections in patients with cancer: results from a large prospective registry. Infection 51, 1153–1159 (2023). https://doi.org/10.1007/s15010-023-02029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-023-02029-4