Abstract
Objectives
To assess the severity of symptoms, duration of infection and viral loads of health-care workers (HCWs) who tested positive for Coronavirus disease 2019 (COVID-19) during Omicron’s prevalence, in regard to vaccination and previous infection.
Methods
During 2 weeks of highest rate of COVID-19 cases in Bosnia and Herzegovina, the positive nasopharyngeal swabs were analysed in 141 HCWs by reverse transcription quantitative PCR, targeting four different genes: RdRp, E, N and nsp14. Uniformed questionnaire was used to collect relevant sociodemographic and epidemiological data from HCWs divided into four groups: unvaccinated/not previously infected (group 1); unvaccinated/previously infected (group 2); vaccinated/not previously infected (group 3); and vaccinated/previously infected (group 4).
Results
We observed that occurrence of fever and smell or taste loss were more frequent in group 1 (86.4% and 25%) and group 3 (76.9% and 19.2%), in comparison to group 2 (64.4% and 6.7%) and group 4 (69.2% and 3.8%), (p = 0.023 and p = 0.003). Although statistically not significant, group 2 (61.9%), group 3 (65.4%), and group 4 (70.8%) experienced negativization within 7 days of positive RT-qPCR test, whereas 51.2% of HCWs from group 1 tested negative later on. There is no significant difference between all four groups regarding Ct values of analysed genes.
Conclusion
During Omicron’s prevalence, the vaccination had less substantial effect on symptomatic disease among HCWs, while fever and loss of smell or taste were considerably less likely to occur upon reinfection. Since viral loads and negativization periods do not seem to significantly vary, irrespective of pre-existing immunity, systemic vaccination and mask-wearing should still be considered among HCWs.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), after more than 2 years of the pandemic, still remains a threat to global health. As expected, various genetic lineages of SARS-CoV-2 have been emerging and circulating globally, resulting in numerous case outbreaks [1]. Following its discovery in South Africa and preliminary evidence suggesting an increased risk of reinfection when compared to previous variants, Omicron was categorized as a variant of concern (VOC) by The World Health Organization (WHO) on November 26, 2021 [2,3,4]. In Bosnia and Herzegovina, the first case was confirmed on December 29, 2021, followed by an unprecedented increase of cases in January of 2022.
Unlike general population, health-care workers (HCWs) are known to be at occupational risk of acquiring the disease, and subsequently, exposing patients and others [5]. However, the nature and scope of this threat remain unclear. In this study, we aimed to assess the symptom intensity, infection duration and viral load of HCWs who tested positive for COVID-19, in regard to vaccination and previous SARS-CoV-2 infection, thus providing supporting data on the possible impact of the pandemic on the health-care system.
Patients and methods
Study population
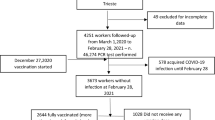
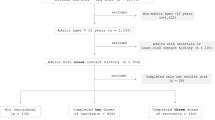
A total of 141 health-care workers (HCWs) at the University Clinical Hospital (UCH) Mostar were enrolled in the study between January 12, 2022 and January 26, 2022. Of these, 52/141 study participants (36.9%) had been fully vaccinated (with 2 doses) with any COVID-19 vaccine approved for use in Bosnia and Herzegovina, whereas 71/141 (50.4%) have reported a previous SARS-CoV-2 infection. Based on these two parameters, the study participants were divided into four groups: unvaccinated/not previously infected (group 1); unvaccinated/previously infected (group 2); vaccinated/not previously infected (group 3); and vaccinated/previously infected (group 4). The enrolment criteria was a SARS-CoV-2 infection confirmed by reverse transcription quantitative PCR (RT-qPCR) from nasopharyngeal swabs.
RT-qPCR
Nasopharyngeal swabs were collected and routinely analysed at the Microbiology department of UCH Mostar using RT-qPCR. The criteria for positive tests was Ct (cycle threshold) value ≤ 38 for both the RdRp (RNA-dependent RNA polymerase) and N (nucleocapsid protein) genes in every given sample. The same swabs were additionally processed at the laboratories of Veterinary Institute of Herzegovina-Neretva Canton, where four genes were analysed, including: RdRp, E (envelope protein), N and nsp14 (non-structural protein 14) gene. RNA was extracted from the samples using Viral RNA extraction kit (Blirt, S.A.) and then subsequently reverse transcribed into complementary DNA (cDNA) using a one-step RT-qPCR assay (qScript XLT One-Step RT-qPCR ToughMix, Quanta Bio), according to the manufacturers’ protocols. Primers and probes targeting the four previously mentioned genes were synthesized and provided by Eurofins genomics (Vienna, Austria). The probes contain FAM (6-carboxyfluorescein) and BBQ (Blackberry Quencher) or TAM. The reaction was performed using magnetic induction cycler (MIC PCR, Bio Molecular Systems) and the results were interpreted using its belonging software, Version 2.10.0.
Ethical statement
All procedures followed were in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ethical approval was acquired from the Ethical Committee at UCH Mostar.
Data collection and statistical analysis
Relevant sociodemographic and epidemiological data were extracted from questionnaires which were filled in by study participants. The questions included: age, gender, profession, vaccination status, type of vaccine, number of administered doses, date of the last vaccination, previous SARS-CoV-2 infection and the date of its occurrence, antibody titers, symptom duration, fever and its duration, clinical manifestations (coughing, sneezing, nasal congestion, loss of smell or taste, sore throat, headache, diarrhoea, vomiting, myalgia and pneumonia), and the dates of positive and negative RT-qPCR tests. The data were processed by IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk, NY). The results regarding categorical variables are presented as absolute numbers (n) and percentages (%). The relationship between variables was determined with the Chi-square test (χ2 test), and all tests were two-tailed, where values of p < 0.05 were considered statistically significant. Quantitative variables i.e. Ct values were presented using boxplot chart.
Results
Population characteristics
During the 2 weeks of highest rate of confirmed COVID-19 cases in Bosnia and Herzegovina since the beginning of the pandemic (January 19, 2022 being the highest peak with 3342 confirmed cases, as reported by WHO), we analysed 141 HCWs from UCH Mostar. Of the total number of study participants, 26 were fully vaccinated and previously infected (group 4). Furthermore, 26 study participants were fully vaccinated and not previously infected (group 3), while 45 were unvaccinated and had recovered from SARS-CoV-2 (group 2). The remaining study participants (46) were neither vaccinated nor previously infected (group 1). Initially, there was a small number of participants who reported receiving a booster dose (n = 10), but no significant influence on our results could be deduced due to the very limited number of such cases. Therefore, we only included HCWs who received 2 doses of any approved vaccine as those “vaccinated” in the study. Additionally, when establishing “previously infected” groups, only RT-qPCR-confirmed cases were taken into consideration, since the reported data regarding antibody titers were scarce and inconsistent. Some of the unvaccinated participants (groups 1 and 2) were non-symptomatic and in contact with an infected person, and therefore were subjected to testing. On the other hand, vaccinated HCWs underwent RT-qPCR testing after the development of symptoms, resulting in no participants from groups 3 and 4 without symptoms.
Participants’ median age was 39 years (range, 21–65 years), with the median of 35.5, 39, 39.5 and 44 years among groups 1, 2, 3 and 4 respectively. Of total 141 HCWs, those 117 (83%) were female, and it is worth noting that out of all the HCWs at UCH Mostar, 73.3% are females, making our study population representative. There was no statistical difference among participants between our four established groups regarding age (p = 0.531) and gender distribution (p = 0.72).
COVID-19 specific symptoms
To assess the impact of COVID-19 vaccination as well as previous recovery from SARS-CoV-2 infection, we compared the incidence of COVID-19 associated symptoms across four groups of study participants. As depicted in Table 1, although not statistically significant, there was a trend of group 1 experiencing longer symptom duration, where 26.8% fall into the ≥ 7 days’ category. Furthermore, we observed that fever, as the most prevalent symptom reported by the study participants, was significantly more frequent in the groups that were not previously infected, irrespective of the vaccination, group 1 (86.4%) and group 3 (76.9%) (p = 0.023). Similar trend can be observed regarding fever duration, with 33.3% of HCWs in the group 1 with no pre-existing immunity experiencing fever for more than 3 days.
We observed no significant difference between study groups regarding COVID-19 associated symptoms: coughing, sneezing, sore throat, nasal congestion, headache, myalgia, diarrhoea and vomiting (Table 1). On the other hand, there was a significant correlation between previous infection and the occurrence of smell or taste loss (p = 0.003). Of note is that, although not statistically significant, pneumonia cases are most prevalent among group 1 (9.1%), while none of the study participants in group 3 developed pneumonia.
Influence of vaccination and previous infection on nasopharyngeal swab SARS-CoV-2 negativization
To further evaluate the impact of vaccination, as well as previous infection on the SARS-CoV-2 viral clearance, we analysed the time required for nasopharyngeal swab negativization (interval between positive and negative RT-qPCR tests). As shown in Fig. 1, negativization period tends to be shorter than 8 days for 70.8% of HCWs within group 4. Following up are group 2 (61.9%) and group 3 (65.4%), with a slightly less percent of people with negativization under 8 days. The only group with more people experiencing negativization after 8 days are those neither vaccinated nor previously infected (group 1) with 51.2%, but without any significant difference.
Analysis of four SARS-CoV-2 genes and their Ct values by RT-qPCR
Laboratories across the world currently utilize several RT-qPCR assays recommended by the WHO, which typically target RdRp, E and N genes. However, it is unknown if results from various tests are equivalent. We aimed to get a broader understanding of 4 different genes (RdRp, E, N, nsp14), and their detection dynamics by comparing Ct value means, as indicators of viral load (Fig. 2). All the participants were tested between third and fifth day of symptom onset or five to seven days after the contact with infected person. There is no significant difference between our four groups regarding Ct values of analysed genes. However, it is evident that E gene tends to be detected in earlier cycles (mean = 26.5), N (mean = 29.6) and nsp14 (mean = 28.6) fairly similarly in between, and RdRp the last (mean = 31). This trend is notable across all four groups.
Discussion
In this study, we found that vaccination had less substantial influence on symptomatic disease, while fever and loss of smell or taste are significantly less likely to occur when a person has previously recovered from COVID-19, irrespective of vaccination. There is also a noticeable trend of participants with no pre-existing immunity experiencing longer symptom duration overall and are more likely to develop pneumonia. Negativization period tends to be the shortest among fully vaccinated and previously infected participants, followed by groups that were either vaccinated or previously infected, suggesting a faster viral clearance in people with vaccine-induced and/or infection-induced immunity. When comparing Ct values of four analysed genes as measures of viral load, we found no significant difference between our groups, indicating similar initial viral load across our study population.
Taking into consideration the time period of our study and high incidence of cases at the time, we assume that the dominant SARS-CoV-2 variant was Omicron (B.1.1.529). WHO classified it as VOC on November 26, 2021, while the first confirmed case in Bosnia and Herzegovina was on December 29, 2021 [1, 2, 6]. Our results regarding clinical manifestations are consistent with previous studies that indicate substantially lower vaccine effectiveness against symptomatic disease caused by the Omicron in comparison to previous variants [7, 8]. Even though their effectiveness is reduced, vaccines do provide adequate levels of protection against symptomatic disease in the first few weeks after administration [9]. However, the majority of our vaccinated participants (82.6%) have received their second dose 5 or more months before enrolment in the study, which might additionally influence the lack of symptom variation among four observed groups. Studies suggest that the Omicron variant is associated with the ability to evade immunity from prior infection [10,11,12]. That is also evident from our study, where 50.4% of participants have reported a previous SARS-CoV-2 infection confirmed by RT-qPCR, of which 90% tested positive 5 or more months before our study. Considering that the large majority of participants had COVID-19 or had been vaccinated with 2 doses well before enrolment in the study, we can assume that their immunity waned over time. Furthermore, our previous study demonstrated sustained titers of SARS-CoV-2 IgG antibodies over one year of infection, implicating that specific neutralizing IgGs are crucial for control of virus spreading rather than sustained IgG titers [13].
Studies showed that the Omicron variant has less impact on the lower respiratory tract, with major clinical manifestations being those of a “mild infection” [14, 15]. This is consistent with our findings, where only 5% of study participants developed pneumonia, and none were hospitalized. However, our study population is generally younger and healthy, ranging from 21 to 65 years of age, and therefore our findings are not indicative of all age groups. According to the latest CDC (Centers for Disease control and Prevention) data, collected during Omicron’s prevalence, unvaccinated adults who are 65 and older are 5.3 times more likely to be hospitalized due to COVID-19, in comparison to their fully vaccinated peers [16]. It is worth noting that we observed 9.1% of participants with no pre-existing immunity (group 1) developing pneumonia, suggesting that either vaccine-induced or infection-induced immunity do play a role in the prevention of serious disease outcome. To expand on the observed trends regarding group 1, it is notable that they experienced longer overall symptom duration (26.8% had symptoms for ≥ 7 days), and fever duration (33.3% had fever for more than 3 days). This directly correlates with longer viral clearance, where more than 50% of HCWs from group of participants without any pre-existing immunity tested negative after 8 or more days. However, it is not yet known how long infective virus persists in patients through different stages of infection, but it has been confirmed that successful SARS-CoV-2 cultivation after 8 days from sampling or symptom onset is possible [17,18,19]. Worth noting is that other participants, regardless of pre-existing immunity, also showcased prolonged viral clearance to some extent. This raises a concern since the latest CDC recommendation shortened isolation time from 10 to 7 days for HCWs, as long as they do not have symptoms [20]. Taking into consideration a relatively low rate of vaccination among HCWs from our study (36.9%), and a significant number of HCWs with no pre-existing immunity (31.2%), we should emphasize on protective equipment utilization in hospital environment. In particular, masks should still be obligatory, regardless of the immune status of HCWs, since Omicron variant is more transmissible when compared to previous variants [1]. This could provide additional protection to HCWs who are at occupational risk of SARS-CoV-2 infection, as well as to patients [5].
Viral load following Omicron infection does not seem to be related to increased infectiousness, as it was the case with previous VOCs [21, 22]. In our study, Ct values were utilized as surrogate markers for the amount of virus in a specific sample. The relevance of Ct value threshold as a measure of potential infectivity is still unclear [17]. We observed that there is no significant difference in Ct values of analysed genes between our four groups, indicating a consistent initial viral load in participants, irrespective of vaccination or previous SARS-CoV-2 infection. Omicron’s immunity evasion may be associated with unusually large number of mutations in the Spike (S) protein, which is responsible for binding and entry into host cells. Relative to the original strain, Omicron has 37 mutations in the S protein, of which 15 are in the receptor-binding domain, which is the target of many neutralizing antibodies [3, 4, 23]. Of note is that previous studies show that the N-gene primer and probe set has a higher specificity compared to that of the RdRp gene, making it one of the best and most accurate targets for SARS-CoV-2 detection [24]. This is in accordance to our findings which suggest that the E gene, followed by N and nsp14, based on the Ct value appearance, present more favourable targets for amplification, in comparison to the RdRp.
Our study showed significant correlation between prior contact with the SARS-CoV-2 and fever onset upon reinfection, irrespective of vaccination status, suggesting that infection-induced immunity could play an important role in the prevention of symptomatic disease. Furthermore, data from the UK show that loss of taste and smell was less commonly reported at the end of December 2021 (16/13%), as compared to the start of December 2021 when Delta variant dominated (44/44%) [25]. Our findings coincide with these data, where 14.2% (20/141) of our study participants reported loss of smell or taste. However, we found that previous infection strongly affected the occurrence of this symptom, irrespective of vaccination, suggesting a stronger upper-respiratory mucosal immunity after natural infection. This may be related to the fact that the current COVID-19 vaccines are administered intramuscularly (systemic vaccination), and primarily induce antibodies of the IgG class, and little to no respiratory IgA [26]. On the other hand, IgA are the most effective antibody class on respiratory mucosae, and several studies have found that they possess superior anti-viral properties vs. IgG for SARS-CoV-2 [27,28,29]. In support of this, hybrid immunity as a result of natural infection and vaccination, could prevent both localized and systemic infection by VOCs, due to significantly larger boost to the neutralizing antibody response compared with 2 doses of vaccine or previous infection alone [30, 31].
This study has several limitations. Small number of participants resulted in difficulty finding significant relationships from the data. Observing a larger group of HCWs should result in a more realistic distinction regarding our study points. Study participants’ median age was 39 years (ranging from 21 to 65), which is not indicative of all age groups, especially older population. Along with that, the majority were females (83%), which although statistically not significant in regard to our study points, still may provide a bias towards certain effects in females. Furthermore, we did not provide comparison between different vaccine types, as there was an overall small percentage of vaccinated participants as a result of low availability of vaccines at the time, and we found no significant correlation between reported vaccine types and any objectives observed in this study (data not shown). Similarly, we only included HCWs who received 2 doses in the study, since there was a very limited number of participants who reported receiving a booster dose, which did not influence our results. Additionally, Omicron variant was only assumed, not identified in each study participant. However, the study period coincides with the highest prevalence of COVID-19 in Bosnia and Herzegovina and the period in which Omicron predominated, after two years of pandemic [32]. Further studies will be needed to confirm which Omicron subvariant caused our local cluster at the time of the study. Finally, although HCWs from our study underwent occasional antibody testing, that does not exclude the possibility that some of the asymptomatic participants seroconverted in the meantime.
Data presented in our study point towards the advantage of having adaptive immunity prior to Omicron infection, and as such contributes to the existing body of evidence on the role of specific immunity against SARS-CoV-2 infections. In conclusion, systemic vaccination of HCWs along with consistent protective equipment utilization might ensure an overall safer environment in health-care facilities.
References
CDC. SARS-COV-2 variant classifications and definitions Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. Accessed 23 Mar 2022
WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Who.int. 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 23 Mar 2022
McCallum M, Czudnochowski N, Rosen LE, Zepeda SK, Bowen JE, Walls AC, et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022. https://doi.org/10.1126/science.abn8652.
Kwon D. Omicron’s molecular structure could help explain its global takeover. Nature. 2022. https://doi.org/10.1038/d41586-022-00292-3.
Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo C-G, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020. https://doi.org/10.1016/S2468-2667(20)30164-X.
Mohapatra RK, Sarangi AK, Kandi V, Azam M, Tiwari R, Dhama K. Omicron (B.1.1.529 variant of SARS-CoV-2); an emerging threat: Current global scenario. J Med Virol. 2022. https://doi.org/10.1002/jmv.27561.
Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2119451.
Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021. https://doi.org/10.1038/d41586-021-03672-3.
GOV. UK. COVID-19 vaccine surveillance report week 4. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf. Accessed 23 Mar 2022
Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022. https://doi.org/10.1056/NEJMc2200133.
Danza P, Koo TH, Haddix M, Fisher R, Traub E, OYong K, et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance - Los Angeles county, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022. https://doi.org/10.15585/mmwr.mm7105e1.
Schubert M, Bertoglio F, Steinke S, Heine PA, Ynga-Durand MA, Maass H, et al. Human serum from SARS-CoV-2-vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant. BMC Med. 2022;20:102.
Šušak B, Mikulić V, Lazarević A, Mikulić I, Arapovic J. Sustained seroprevalence of SARS-CoV-2 antibodies one year after infection: one of the first COVID-19 cluster cases in Bosnia and Herzegovina. Bosn J Basic Med Sci. 2022. https://doi.org/10.17305/bjbms.2021.6340.
Maisa A, Spaccaferri G, Fournier L, Schaeffer J, Deniau J, Rolland P, et al. First cases of Omicron in France are exhibiting mild symptoms, November 2021-January 2022. Infect Dis Now. 2022. https://doi.org/10.1016/j.idnow.2022.02.003.
Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021. https://doi.org/10.26355/eurrev_202112_27652.
CDC. COVID data tracker. Centers for Disease Control and Prevention. 2022. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalizations-vaccination. Accessed 16 May 2022
Platten M, Hoffmann D, Grosser R, Wisplinghoff F, Wisplinghoff H, Wiesmüller G, et al. SARS-CoV-2, CT-values, and infectivity-conclusions to be drawn from side observations. Viruses. 2021. https://doi.org/10.3390/v13081459.
Sun J, Zhu A, Li H, Zheng K, Zhuang Z, Chen Z, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect. 2020. https://doi.org/10.1080/22221751.2020.1760144.
Abe T, Ikeda T, Tokuda Y, Ito J, Suzuki Y, Narahara C, et al. A patient infected with SARS-CoV-2 over 100 days. QJM. 2021. https://doi.org/10.1093/qjmed/hcaa296.
CDC. Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed 24 Mar 2022
Hay JA, Kissler SM, Fauver JR, Mack C, Tai CG, Samant RM, et al. Viral dynamics and duration of PCR positivity of the SARS-CoV-2 Omicron variant. BioRxiv. 2022. https://doi.org/10.1101/2022.01.13.22269257.
Sentis C, Billaud G, Bal A, Frobert E, Bouscambert M, Destras G, et al. SARS-CoV-2 Omicron variant, lineage BA.1, is associated with lower viral load in nasopharyngeal samples compared to delta variant. BioRxiv. 2022. https://doi.org/10.1101/2022.02.02.22269653.
Kandeel M, Mohamed MEM, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS. Omicron variant genome evolution and phylogenetics. J Med Virol. 2022. https://doi.org/10.1002/jmv.27515.
Abbasi H, Tabaraei A, Hosseini SM, Khosravi A, Nikoo HR. Real-time PCR CT value in SARS-COV-2 detection: RDRP or N gene? Infection. 2021. https://doi.org/10.1007/s15010-021-01674-x.
Vihta K-D, Pouwels KB, Peto TEA, Pritchard E, House T, Studley R, et al. Omicron-associated changes in SARS-CoV-2 symptoms in the United Kingdom. BioRxiv. 2022. https://doi.org/10.1101/2022.01.18.22269082.
Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–8.
Focosi D, Maggi F, Casadevall A. Mucosal vaccines, sterilizing immunity, and the future of SARS-CoV-2 virulence. Viruses. 2022. https://doi.org/10.3390/v14020187.
Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claër L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abd2223.
Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abf1555.
Bates TA, McBride SK, Leier HC, Guzman G, Lyski ZL, Schoen D, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022. https://doi.org/10.1126/sciimmunol.abn8014.
Vicenti I, Gatti F, Scaggiante R, Boccuto A, Zago D, Basso M, et al. The second dose of the BNT162b2 mRNA vaccine does not boost SARS-CoV-2 neutralizing antibody response in previously infected subjects. Infection. 2022. https://doi.org/10.1007/s15010-021-01680-z.
Arapović J, Skočibušić S. The first two months of the COVID-19 pandemic in Bosnia and Herzegovina Single-center experience. Bosn J Basic Med Sci. 2020;20(3):396–400.
Acknowledgements
We thank Dr. Maja Ostojić, Dr. Sanja Jakovac, Dr. Jadranka Nikolić, Dr. Svjetlana Grgić, Dr. Doris Martinović, s. Zdravka Marić, and Ms. Anela Džidić for helping in collecting of samples. Special thanks to the International Atomic Energy Agency for their generous donation, which allowed us to perform molecular testing. We thank all the HCWs at UCH Mostar who participated in the study.
Funding
International Atomic Energy Agency, INT0098 technical cooperation project, Maja Arapović
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study, were involved in the interpretation of the data, and the development and approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Laura, L., Dalmatin-Dragišić, M., Martinović, K. et al. Does pre-existing immunity determine the course of SARS-CoV-2 infection in health-care workers? Single-center experience. Infection 51, 323–330 (2023). https://doi.org/10.1007/s15010-022-01859-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01859-y