Abstract
Purpose
To assess the association between vitamin D (VD) supplementation and the risk of lower respiratory tract infection (LRTI) among infants.
Methods
This is a nested case–control study from an ongoing prospective birth cohort in Wuhan from 2013. Cases were subjects free of neonatal pneumonia but later developed LRTI during infancy, who were matched with five randomly selected controls by infant sex, birth year, and birth season. We included 190 cases and 950 controls in the final analysis. The primary outcome was the first LRTI incident and the exposure was VD supplementation from birth to the index endpoint. The association between VD supplementation and LRTI risk was assessed using the Cox proportional-hazards regression model.
Results
Infants taking supplements had a 59% relative reduction in the hazard ratio of LRTI (HR = 0.41; 95% CI 0.26, 0.64) compared to those not supplemented. There was a linear relationship between LRTI risk and VD supplementation within range of 0–603 IU/day: for each 100 IU per day increment in VD supplementation, infants experienced a 21% lower risk of developing LRTI (adjusted HR: 0.79; 95% CI 0.71, 0.89). The linear relationship was stably observed in the sensitivity analyses as well.
Conclusions
VD supplementation was associated with the reduced risk of LRTI throughout infancy, and the optimal supplementation dose for infants may be beyond the current recommendation.
Similar content being viewed by others
Introduction
Lower respiratory tract infection (LRTI) has long been a leading cause of morbidity and mortality in infancy [1, 2]. It was estimated to be responsible for about 16.4 million episodes of hospital admissions in 2015 and 0.65 million deaths in 2016 among children younger than 5 years, of which more than half were infant cases [3, 4]. Infancy is a key early period in the life course, and poor wellbeing during such period can not only impact the present health and development but also their later life [5]. Given that, any promising strategies for the prevention of LRTI should be explored as extensively and early as possible.
Vitamin D (VD) has been shown to possess immunomodulatory properties and mediate the immune responses to infections [6,7,8]. Mounting epidemic evidence has linked VD deficiency to the significantly increased risk of LRTI in infants [9,10,11] and young children [12,13,14], implying a potential benefit of VD in the prevention and treatment of LRTI in early childhood. Unfortunately, due to the inadequate maternal VD intake and accompanying marginal levels of VD, VD deficiency is prevalent among neonates worldwide, with prevalence ranging from 30% in America to 90–96% in China and South-East Asia [15, 16]. Moreover, breast milk contains only about 40 IU/L of VD which is far from sufficient to satisfy the needs of infants [17], as a result, infants will be chronically deprived of VD nutritional status and prone to the increased risk of LRTI. There has been a growing concern about the role of VD supplementation in preventing childhood LRTI [18]. However, no definite answer can be given due to limited evidence.
Previously, we noted a beneficial role of VD supplementation in reducing LRTI risk within the first 6 months of life [19]. Therefore, we conducted this specially designed nested case–control study, to further examine whether VD supplementation is associated with the risk of LRTI throughout infancy, and on this basis, to further assess the optimal supplementation dose to prevent LRTI.
Materials and methods
Study design and data collection
We conducted this nested case–control study, selecting the subjects from mother–infant pairs included in the Tongji Maternal and Child Health Cohort (TMCHC). The TMCHC is an ongoing prospective birth cohort study with the recruitment of pregnant women from 2013 to 2017 in Wuhan, China, to evaluate the impact of nutritional, environmental, and lifestyle exposures on the health status of the mother and their offspring [19,20,21]. Baseline information with demographic characteristics (e.g., birth date, education level, and household income), anthropometric variables (e.g., weight and height before pregnancy), lifestyles (e.g., smoking status), and obstetric history were obtained at the enrollment via face-to-face interview. After childbirth, data on birth information (e.g., birth date, sex of infant and birth weight, birth length, congenital illness) was extracted from health medical records. Postpartum follow-up visits were administered via structured questionnaires by trained investigators at 1 month, 3 months, 6 months, and 12 months, respectively, to track the health data of mother and their babies. All procedures performed in these studies involving human participants were approved by the ethics review committee of Tongji Medical College of Huazhong University of Science and Technology (No. 201302). All participants provided written informed consent upon enrollment.
Study population
Initially, 2260 infants from TMCHC who had completed 1-, 3-, 6-, 12-month follow-up visits were included in the study. Of these participants, 358 infants were excluded for the ineligibility: 248 infants for preterm or post-term (gestational age of < 37 or ≥ 42 weeks), low or high birthweight (< 2500 g or ≥ 4000 g), 63 infants for congenital illnesses; 47 infants for neonatal pneumonia; 181 infants for missing data on feeding, supplements use, and illness. After these exclusions, 1721 participants were eligible for this study, of whom we screened 190 infants who developed bronchitis, bronchiolitis, and pneumonia as the incident case of LRTI. For each case of LRTI, five controls were randomly matched for sex, birth year, and birth season. The final analysis consists of 190 cases and 950 controls (Fig. 1).
Flow diagram for the inclusion of 190 cases with lower respiratory tract infection and 950 controls based on a nested case–control design from Tongji Maternal and Child Health Cohort (TMCHC) project. Definition of abbreviations: LRTI, lower respiratory tract infection; TMCHC, Tongji Maternal and Child Health Cohort
Outcome assessment
LRTI was defined as pediatrician-diagnosed bronchitis or bronchiolitis or pneumonia. The primary outcome endpoint was the first LRTI incident, the occurrence time of which was used to establish the index time for the controls.
Assessment of VD supplementation
VD supplementation details, including brands of the supplements, dosage, duration, and weekly frequency of use (days/week), were first recorded at 1-month postnatal visit then repeatedly tracked at 3 months, 6 months, and 12 months via the constructed questionnaires. For each case and their matched controls, the average VD intake from supplementation (IU/day) from birth to the endpoint was evaluated by dividing the total amounts of consumption by the total number of days during this period. The infants were dichotomized according to whether taking supplements or not and further classified into quartiles according to their VD intake ranking.
Covariate assessment
Baseline demographic and clinical characteristics were considered as potential confounders, including maternal age at delivery (25, 25–34, ≥ 35), maternal education level (0–9, 10–12, > 12 years); household income (< 5000, 5000–9999, ≥ 10,000 CNY/month), pre-pregnancy body mass index (BMI) which was calculated as weight in kilograms divided by height squared in meters and categorized as < 18.5, 18.5–23.9, ≥ 24 kg/m2 according to the Chinese adult BMI classification [22], parental smoking status (yes or no); upper respiratory tract infection (URTI) before endpoint (yes or no), parity (1, ≥ 2), infant sex (male or female), the birth season (spring: March–May, summer: June–September, fall: September–November, and winter: December–February), birth weight and birth length. Besides, formula consumption before the outcome endpoint was also an important confounder to be controlled. Details of formula consumption (addition time, formula volume) were repeatedly assessed through the follow-up questionnaires. The average daily formula consumption from birth to the index endpoint was calculated by dividing the total amounts of consumption by the total number of days during this period, categorized as 0, 1–249, 250–499, ≥ 500 mL/day. Within this specifically designed study, the feeding pattern was categorized into fully breastfeeding and formula-feeding based on the calculated formula consumption during the period from birth to the index endpoint using the cutoff of zero.
Statistical analysis
Continuous variables were reported as mean (SD) and medians with IQRs (interquartile ranges). Categorical variables were reported as n (%). Group differences were compared using the Student t test or Wilcoxon rank-sum test for continuous variables and chi-square test for categorical variables. To account for the occurrence time, Cox proportion hazards regression models were performed to assess the association between VD supplementation and the risk of LRTI by estimating hazard ratios (HRs) and 95% CIs. Analyses for association were both performed by treating VD intake from the supplements as a categorical variable categorized into quartiles, considering the lowest quartile as the reference category, and as a continuous variable using a unit of 100 IU/day. First step adjustments were made for feeding pattern and parity which was shown with the statistical difference between groups. Further adjustment was made to control for demographic characteristics and other potential factors, including maternal age at delivery, education levels, household income, birthweight, maternal prepregnancy BMI, parental smoking status before pregnancy, formula consumption, and URTI history [23, 24]. Tests of linear trend across increasing categories of adherence were conducted by assigning the median value to each category and treating it as a continuous variable. The association between VD supplementation and risk of LRTI was also checked graphically using restricted cubic splines with the reference dose defined as 400 IU/day. Accounting for the potential effect of feeding patterns, the association was examined separately in fully breastfed infants and formula-fed infants. In addition, to examine the robustness of our results, we run the subgroup analyses based on other potential confounders including infant sex and the season of birth. We also examined whether estimates were changed when the study population was restricted to infants with no siblings and no URTI history. To address the bias by unmeasured confounders, we ran a bias analysis referring to the previous study by Pasternak et al. [25] by constructing a hypothetical unmeasured confounder which was considered to increase the risk of LRTI by a factor of 1.5–5.0 and which has a 1–2 times higher proportion in the unsupplemented infants (with proportions varying from 10 to 90%) than the supplemented infants (varying from 5% to 45%). Statistical analysis was performed using the statistical program R, version 4.0.2. P value < 0.05 was considered statistically significant.
Results
In total, 190 cases with LRTI and 950 controls were recruited in the present study. The demographic and other relevant characteristics of the recruited participants are shown in Table 1. The median average consumption of formula was 75 mL/day, and 26.3% did not receive formula before the LRTI incident; 37.6% had a low average intake of less than 250 mL/day. Infants who had siblings were more likely to develop LRTI than those without. There were no significant differences in maternal age at delivery, education levels, household income, pre-pregnancy BMI, parental smoking, infant sex, birthweight, birth length, birth season, URTI history, and formula consumption between the cases and their controls. Most infants (1034 out of 1140) had taken the VD supplements before the LRTI incident (Table 2). The median average intake of VD from supplements was 250 IU/day (Table 1).
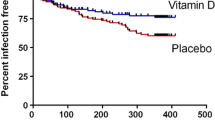
In the multivariate cox regression analysis relating supplementation to LRTI with regard to occurrence time, we observed a 59% reduction in HR for infants who received VD supplementation compared to those not supplemented (adjusted HR = 0.41; 95% CI: 0.26, 0.64) (Table 2). LRTI risk decreased linearly with the increasing supplementation quartile, (p trend < 0.001), and the multivariate HR for better adherence to supplementation (the highest quartile of VD supplementation) was 0.47 (95%CI: 0.31,0.73) as compared with the lowest quartile (Table 2). Similarly, when analyzing VD supplementation as a continuous variable, the risk of LRTI also decreased linearly with increasing supplementation dose (p for linear association < 0.001) (Fig. 2), and a 100 IU per day increase in VD supplementation was associated with a 21% reduction in LRTI risk (adjusted HRs = 0.79; 95%CI:0.71,0.89) (Table 2). Furthermore, the linearity of VD supplementation with LRTI risk is independent of infant feeding (Fig. 3).
Fitted curve for hazard ratio (HR) and 95% confidence interval (CI) of lower respiratory tract infection according to average daily VD intake from supplements (IU/d) (N = 1140). Definition of abbreviations: HR, hazard ratio; CI, confidence interval; LRTI, lower respiratory tract infection; VD, vitamin D. The tinted region indicates 95% confidence intervals around the hazard risk
Subgroup analysis of association between VD supplementation and LRTI risk, stratified by feeding pattern (N = 1140). Definition of abbreviations: VD, vitamin D; LRTI, lower respiratory tract infection; HR, hazard ratio; CI, confidence interval. a The HRs were adjusted for maternal age, education levels, household income, parity, sex, birth season, birthweight, maternal pre-pregnancy body mass index, parental smoking before pregnancy, and history of upper respiratory tract infection; b the HRs were further adjusted for formula consumption
Sensitivity and bias analyses
In analyses stratified by infant sex and birth season, the associations between VD supplementation and LRTI risk were consistent with those in the primary analysis (Figs. 4, 5). Likewise, the dose–response relationship between every 100 IU/d increase in VD intake from supplements and the reduced risk of LRTI remained unchanged in the analysis restricted to infants with no siblings and no history of URTI (Fig. 6). In the bias analysis adjusting for the unmeasured confounder, even if the prevalence of this confounder was 90% and 45% in the unsupplemented and supplemented groups, respectively, and the risk estimate of LRTI for such a confounder was as high as fivefold, infants in the supplemented group still had a 33% reduction in the HR of LRTI (Table S1).
Subgroup analysis of association between VD supplementation and LRTI risk, stratified by infant sex (N = 1140). Definition of abbreviations: VD, vitamin D; LRTI, lower respiratory tract infection; HR, hazard ratio; CI, confidence interval. a The HRs were adjusted for maternal age, education levels, household income, parity, birth season, birthweight, maternal pre-pregnancy body mass index, parental smoking before pregnancy, formula consumption and history of upper respiratory tract infection
Subgroup analysis of association between VD supplementation and LRTI risk, stratified by the season of birth (N = 1140). Definition of abbreviations: VD, vitamin D; LRTI, lower respiratory tract infection; HR, hazard ratio; CI, confidence interval. a The HRs were adjusted for maternal age, education levels, household income, parity, sex, birthweight, maternal pre-pregnancy body mass index, parental smoking before pregnancy, formula consumption and history of upper respiratory tract infection
Analysis of association between VD supplementation and LRTI risk, restricted to infants of no siblings and no URTI history (N = 687). Definition of abbreviations: VD, vitamin D; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection; HR, hazard ratio; CI, confidence interval. a The HRs were adjusted for maternal age, education levels, household income, sex, birth season, birthweight, maternal pre-pregnancy body mass index, parental smoking before pregnancy, and formula consumption
Discussion
In this nested case–control study, we found that infants with VD supplementation had a 59% lower risk of LRTI as compared with those not supplemented, and the risk of LRTI decreased linearly within an intake range of 0–603 IU/day from supplements. These results were robust in the sensitivity analyses considering the potential cofounders, such as infant sex, birth season, and feeding patterns, as well as by restricting to infants who had no siblings and no URTI history.
To our knowledge, there has been one study among infants [18] and three studies among young children [26,27,28] assessing the association between VD supplementation and LRTI risk but demonstrating inconsistent results. Consistent with our findings, Sigh et al. and Leis et al. [27, 28] found the intake of VD in the week prior to LRTI in children with LRTI was lower than that of the control group, and children with an intake of less than 80 IU/kg/day had a nearly 4-times increase in LRTI risk as compared with VD intake of greater than 80 IU/kg/day. According to World Health Organization (WHO) growth standards, the 50th percentile of infant weight is approximately 5 kg by 2 months of age and 9 kg by 1 year of age, which means that the optimal VD intake for infants may be more than 400 IU considering LRTI risk [29]. Likewise, we also noticed the LRTI risk reduced linearly within the supplementation dose range of 0–603 IU/day among infants. The authors suspected that the property of VD in promoting lung development [30] and enhancing the innate immune response to infection [31, 32] may be the underlying mechanism. In contrast, Manaseki-Holland et al. [18] and Gupta et al. [26] observed a null effect of VD supplementation on the incidence of pneumonia in healthy infants and the incidence of recurrent pneumonia in children with pneumonia. These inconsistencies could likely be attributed to the difference in dose regimen (a quarterly dose vs. daily dose) and outcome indicator (pneumonia vs. overall LRTIs including bronchitis and pneumonia). Considering such aspects, Martineau et al. conducted a system meta-analysis using individual participant data from 25 randomized controlled trials and reported a benefit of VD supplementation in the prevention of RTI including URTI and LRTI [33], unfortunately, Martineau et al. failed to determine the optimal supplementation dose [33].
There are nearly 90% of infants born with VD deficiency worldwide [15]. Although infants could compensate for the demands of VD via the formula that was fortified with a content of about 400 IU/L of VD [34], our data showed that in contrast to a median VD intake of 250 IU/day from supplements, the median formula consumption was only 75 mL/day (equivalent to 30 IU/day of VD), and as a result, far from meeting infants’ needs. On the other hand, infants are unlikely to obtain VD from skin synthesis as they were usually avoided from direct sun exposure in case of damage to the eyes and skin [34]. Taken all together, VD supplements seem to be the main source as well as the optimal source of VD for infants. In the past two decades, a dose of 400 IU/day of VD has been recommended for infants regardless of the feeding pattern and sun exposure with aim of preventing rickets [34]. However, in terms of additional LRTI risk, our findings, in parallel with the study by leis et al., suggest that the daily dose of 400 IU may not be sufficient, and future studies on a larger dose in LRTI prevention were warranted. Besides, the COVID-19 pandemic has reached an unprecedented scale worldwide, and children can be infected with the risk of developing mild and moderate pneumonia [35], especially infants [36]. There is no vaccine available for infants so far, in such a case, our study may provide a possible prevention strategy for LRTI in young children, which also warrants future research.
In this study, the main strength was its prospective nature, because the assessment of participants’ supplements use was prior to the LRTI incident, making it possible to establish a causal relationship. The second strength of our study includes a sufficient sample size that would reduce casual bias. Third, the VD supplements were taken routinely at a daily dose, which may be more conducive to maintaining stable serum VD levels than a single large dose. This study also has some limitations. First, there is a possibility of uncontrolled confounding, for example, the household crowded, and immunization status. However, this may have a limited impact on our study given the high possibility of unadjusted confounders being randomly distributed among the study population. The risk estimate of LRTI remained reduced after the adjustment for the unmeasured confounder in the bias analysis as well. Also, the robustness of the association was confirmed in a number of sensitivity analyses considering the major potential confoundings. A second limitation is mother-reported data; however, we have repeatedly instructed the mothers on how to report the infant's health information at each follow-up visit so that our results may be less likely to be impacted by the recall bias and misclassification. Third, the VD status at birth was not measured in this study, although about 90% of infants remained with VD deficiency or insufficiency at birth in China [16], the deficient degree may vary, as a result, we were unable to answer the question whether the effect size of VD supplementation on LRTI will vary by different degrees of deficiency, which deserves future research. Finally, VD binding protein polymorphisms (VDBP) genotypic variability is a significant factor in determining VD status associated with VD intake [37]. In our study, the participants were mainly of Han ethnicity, among whom the most common genotype is the VDBP 1S allele [38], which was associated with higher VD status when receiving VD intake than other alleles [37], thus making it a limitation when generalized to race/ethnicity of other alleles.
Conclusions
In this study, we found a significant inverse dose–response association between VD intake from supplementation and the risk of developing LRTI throughout infancy. Our finding provides preliminary evidence that the VD supplementation may be a possible strategy to prevent LRTI, and the optimal supplementation dose may be beyond the current recommendation, which is worth confirming further.
Data availability
The data set used in the study is available from the corresponding author on reasonable request.
References
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–35.
Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–90.
McAllister DA, Liu L, Shi T, Chu Y, Reed C, Burrows J, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7:e47–57.
Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–210.
McEniry M, Palloni A, Dávila AL, Gurucharri AG. Early life exposure to poor nutrition and infectious diseases and its effects on the health of older Puerto Rican adults. J Gerontol B Psychol Sci Soc Sci. 2008;63:S337–48.
Chirumbolo S, Bjorklund G, Sboarina A, Vella A. The role of vitamin d in the immune system as a pro-survival molecule. Clin Ther. 2017;39:894–916.
Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–6.
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3.
Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, et al. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71.
Erol M, Kaya H, BostanGayret Ö, Yiğit Ö, Hamilçıkan Ş, Can E. The Effect of Vitamin D Deficiency on the Severity of Bronchiolitis in Infants. J Pediatr Res. 2017;4:12–6.
Lai SH, Liao SL, Tsai MH, Hua MC, Chiu CY, Yeh KW, et al. Low cord-serum 25-hydroxyvitamin D levels are associated with poor lung function performance and increased respiratory infection in infancy. PLoS ONE. 2017;12: e0173268.
Roth DE, Shah R, Black RE, Baqui AH. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet. Bangladesh Acta Paediatr. 2010;99:389–93.
Velarde López AA, Gerber JS, Leonard MB, Xie D, Schinnar R, Strom BL. Children with lower respiratory tract infections and serum 25-hydroxyvitamin D3 levels: A case-control study. Pediatr Pulmonol. 2016;51:1080–7.
Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–7.
Saraf R, Morton SM, Camargo CA Jr, Grant CC. Global summary of maternal and newborn vitamin D status – a systematic review. Matern Child Nutr. 2016;12:647–68.
Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013;29:953–7.
Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10:154–62.
Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. The Lancet. 2012;379:1419–27.
Hong M, Xiong T, Huang J, Wu Y, Lin L, Zhang Z, et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern Child Nutr. 2020;16: e12987.
Huang L, Chen X, Zhang Y, Sun G, Zhong C, Wang W, et al. Gestational weight gain is associated with delayed onset of lactogenesis in the TMCHC study: A prospective cohort study. Clin Nutr. 2019;38:2436–41.
Li Q, Xu S, Chen X, Zhang X, Li X, Lin L, et al. Folic acid supplement use and increased risk of gestational hypertension. Hypertension. 2020;76:150–6.
Zhou BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96.
Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, Zar HJ, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta–analysis. J Glob Health. 2015;5(2):020416
Simoes EAF. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:118–26.
Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814–23.
Gupta P, Dewan P, Shah D, Sharma N, Bedi N, Kaur IR, et al. Vitamin D Supplementation for Treatment and Prevention of Pneumonia in Under-five Children: A Randomized Double-blind Placebo Controlled Trial. Indian Pediatr. 2016;53:967–76.
Leis KS, McNally JD, Montgomery MR, Sankaran K, Karunanayake C, Rosenberg AM. Vitamin D intake in young children with acute lower respiratory infection. Transl Pediatr. 2012;1:6–14.
Singh N, Kamble D, Mahantshetti NS. Effect of Vitamin D Supplementation in the Prevention of Recurrent Pneumonia in Under-Five Children. Indian J Pediatr. 2019;86:1105–11.
Patorno E, Huybrechts KF, Bateman BT, Cohen JM, Desai RJ, Mogun H, et al. Lithium Use in Pregnancy and the Risk of Cardiac Malformations. N Engl J Med. 2017;376:2245–54.
Lykkedegn S, Sorensen GL, Beck-Nielsen SS, Christesen HT. The impact of vitamin D on fetal and neonatal lung maturation A systematic review. Am J Physiol Lung Cell Mol Physiol. 2015;308:L587-602.
Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–39.
Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20.
Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2):1–44.
Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145: e20200702.
Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–61.
Newton DA, Baatz JE, Kindy MS, Gattoni-Celli S, Shary JR, Hollis BW, et al. Vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr Res. 2019;86:662–9.
Shao B, Jiang S, Muyiduli X, Wang S, Mo M, Li M, et al. Vitamin D pathway gene polymorphisms influenced vitamin D level among pregnant women. Clin Nutr. 2018;37:2230–7.
Acknowledgements
The authors sincerely acknowledge all participants and staff in the TMCHC project for their valuable contributions.
Funding
This study is supported by National Program on Basic Research Project of China (NO.2013FY114200), the National Natural Science Foundation of China (No. 82173513).
Author information
Authors and Affiliations
Contributions
HM collected data and performed the data analyses and drafted the manuscript. XT, HJ, WY, LL, ZZ, GQ, HL, WH, and GD participated in data collection and validation, and helped review and edit the manuscript. Profs. HL, YN, YX conceptualized and designed the study, co-ordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors gave final approval of the work to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Ethical approval
All procedures performed in these studies involving human participants were approved by the ethics review committee of Tongji Medical College of Huazhong University of Science and Technology (No. 201302).
Consent to participate
All participants provided written informed consent upon enrollment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong, M., Xiong, T., Huang, J. et al. Vitamin D supplementation and lower respiratory tract infection in infants: a nested case–control study. Infection 51, 109–118 (2023). https://doi.org/10.1007/s15010-022-01845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01845-4