Abstract
Purpose
The value of follow-up blood culture (FUBC) in Gram-negative bacteremia (GNB) management is controversial. We evaluated bedside risk predictors and their probabilities of yielding positive FUBCs in GNB.
Methods
All adult patients with GNB in a 2700-bed tertiary center were retrospectively enrolled between January 2019 and December 2019. Only one initial GNB episode was included per patient. Positive FUBC was defined as isolation of the same organism in blood culture 48–72 h after the initial blood culture.
Results
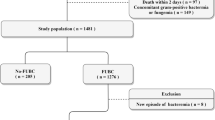
A total of 2216 patients with GNB were identified, of whom 34.4% underwent FUBC. Of the 645 patients with FUBCs analyzed in the study, 89 (13.8%) had positive FUBCs. In multivariate analysis, hemodialysis [adjusted odds ratio (aOR), 2.6], fever on the day of FUBCs (aOR 3.6), intravascular device (aOR 2.4), no use of in vitro active antibiotic within 24 h (aOR 2.5), non-fermenting bacteria (aOR 4.7), and multidrug resistance (aOR 5.4) were independent risk factors for positive FUBCs. If microbiological results were excluded in multivariate analysis, hemodialysis, immunosuppressive treatment, fever on the day of FUBCs, and intravascular device were independent bedside risk predictors for positive FUBCs. The yield of FUBCs increased from 3.0% (95% CI 1.0–7.0) to 63.6% (95% CI 25.6–100) as the number of bedside risk predictors increased from 0 to 4. In addition, positive FUBCs were significantly associated with 30 day mortality.
Conclusions
FUBCs may not need to be routinely used for patients with GNB bacteremia, and bedside risk predictors could be helpful in identifying patients for whom FUBC is likely to be useful.

Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17.
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-55.
Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62:e1-50.
Chong YP, Moon SM, Bang KM, et al. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother. 2013;57:1150–6.
Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis. 2016;16:286.
Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis. 2017;65:1776–9.
Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019;69:1091–8.
von Dach E, Albrich WC, Brunel AS, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7 day treatment, or 14 day treatment on 30 day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323:2160–9.
Mitaka H, Gomez T, Lee YI, Perlman DC. Risk factors for positive follow-up blood cultures in gram-negative bacilli bacteremia: implications for selecting who needs follow-up blood cultures. Open Forum Infect Dis. 2020;7:ofaa110.
Giannella M, Pascale R, Pancaldi L, et al. Follow-up blood cultures are associated with improved outcome of patients with gram-negative bloodstream infections: retrospective observational cohort study. Clin Microbiol Infect. 2020;26:897–903.
Maskarinec SA, Park LP, Ruffin F, et al. Positive follow-up blood cultures identify high mortality risk among patients with Gram-negative bacteraemia. Clin Microbiol Infect. 2020;26:904–10.
Chan JD, Ta A, Lynch JB, Bryson-Cahn C. Follow-up blood cultures in E. coli and Klebsiella spp. bacteremia-opportunities for diagnostic and antimicrobial stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:1107–11.
Kang CK, Kim ES, Song KH, et al. Can a routine follow-up blood culture be justified in Klebsiella pneumoniae bacteremia? A retrospective case-control study. BMC Infect Dis. 2013;13:365.
Shi H, Kang CI, Cho SY, Huh K, Chung DR, Peck KR. Follow-up blood cultures add little value in the management of bacteremic urinary tract infections. Eur J Clin Microbiol Infect Dis. 2019;38:695–702.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin Infect Dis. 2009;49:1–45.
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:427–31.
Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56-93.
Wayne PA. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing: 31st informational supplement M100-S31 2021.
Jung J, Song KH, Jun KI, et al. Predictive scoring models for persistent gram-negative bacteremia that reduce the need for follow-up blood cultures: a retrospective observational cohort study. BMC Infect Dis. 2020;20:680.
Spaziante M, Oliva A, Ceccarelli G, Alessandri F, Pugliese F, Venditti M. Follow-up blood cultures in Gram-negative bacilli bacteremia: are they needed for critically ill patients? Minerva Anestesiol. 2020;86:498–506.
Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–7.
Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39:2206–12.
Tabriz MS, Riederer K, Baran J Jr, Khatib R. Repeating blood cultures during hospital stay: practice pattern at a teaching hospital and a proposal for guidelines. Clin Microbiol Infect. 2004;10:624–7.
Funding
This study was supported by a grant (grant number: 2021IL0042) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Author information
Authors and Affiliations
Contributions
Conceptualization: HK and YPC. Methodology: HK and YPC. Data curation: HK, HS, HC, and SP. Software, formal analysis: HK. Writing—original draft: HK. Writing—review and editing: YPC. Supervision: HS, M-NK, SB, JJ, MJK, S-HK, S-OL, S-HC, and YSK. Funding acquisition: YPC.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report conflicts of interest. All the authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Ethics approval
This study had no human or animal participants, and it is not required ethical approval and informed consent for this type.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H., Seo, H., Chung, H. et al. Bedside risk prediction for positive follow-up blood culture in Gram-negative bacilli bacteremia: for whom is follow-up blood culture useful?. Infection 50, 689–697 (2022). https://doi.org/10.1007/s15010-021-01742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01742-2