Abstract
Background
The ISARIC prospective multinational observational study is the largest cohort of hospitalized patients with COVID-19. We present relationships of age, sex, and nationality to presenting symptoms.
Methods
International, prospective observational study of 60 109 hospitalized symptomatic patients with laboratory-confirmed COVID-19 recruited from 43 countries between 30 January and 3 August 2020. Logistic regression was performed to evaluate relationships of age and sex to published COVID-19 case definitions and the most commonly reported symptoms.
Results
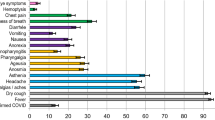
‘Typical’ symptoms of fever (69%), cough (68%) and shortness of breath (66%) were the most commonly reported. 92% of patients experienced at least one of these. Prevalence of typical symptoms was greatest in 30- to 60-year-olds (respectively 80, 79, 69%; at least one 95%). They were reported less frequently in children (≤ 18 years: 69, 48, 23; 85%), older adults (≥ 70 years: 61, 62, 65; 90%), and women (66, 66, 64; 90%; vs. men 71, 70, 67; 93%, each P < 0.001). The most common atypical presentations under 60 years of age were nausea and vomiting and abdominal pain, and over 60 years was confusion. Regression models showed significant differences in symptoms with sex, age and country.
Interpretation
This international collaboration has allowed us to report reliable symptom data from the largest cohort of patients admitted to hospital with COVID-19. Adults over 60 and children admitted to hospital with COVID-19 are less likely to present with typical symptoms. Nausea and vomiting are common atypical presentations under 30 years. Confusion is a frequent atypical presentation of COVID-19 in adults over 60 years. Women are less likely to experience typical symptoms than men.
Similar content being viewed by others
Background
Despite the pandemic’s immense human cost, enormous economic toll, and extensive research response, the precise clinical characteristics of COVID-19 remain unclear [1]. At the start of the outbreak, COVID-19 was broadly characterised as a severe respiratory illness presenting with fever, cough and an atypical pneumonia [2,3,4,5]. Altered sense of taste and smell have since been found to be strongly associated with the disease [6, 7]. However, a review of 77 observational studies found substantial proportions of patients presenting with less typical symptoms [8].
Different sets of clinical criteria for suspected COVID-19 have been produced by the World Health Organization (WHO) [9], Centers for Disease Control and Prevention in the United States [10], Public Health England [11], and the European Centre for Disease Prevention and Control [12] (Table 1). Defining presenting symptoms of COVID-19 is further complicated by clinical experience suggesting that patients frequently present with atypical symptoms other than cough, fever and shortness of breath. This variation in the clinical characterisation of COVID-19 is problematic, as case definitions are used to guide clinical diagnosis, disease surveillance, and public health interventions.
The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) cohort study is an international collaboration to gather reliable observational data about patients admitted to hospital with COVID-19 [13]. Here, we present an analysis of how symptoms of patients admitted to hospital with confirmed COVID-19 vary by age and sex. Secondarily, we investigated how sensitivity of clinical case definitions varied among these populations, and explored heterogeneity among countries.
Methods
Study design and setting
This analysis used international observational data of clinical features of patients admitted to hospital with COVID-19 between 30 January and 3 August 2020. The ISARIC/WHO Clinical Characterisation Protocol for Severe Emerging Infections is a standardized protocol for investigation of severe acute infections by pathogens of public health interest [14]. ISARIC case report forms [15] allow standardized data collection from the start of an outbreak and rapid dissemination of clinical information [3, 16,17,18,19,20]. Different tiers of data collection exist to allow sites to collect data to the highest possible standards while recognising varying levels of resource for data collection during epidemics. Details of symptoms at admission were included on the case report forms for all tiers. Data were collected via electronic ‘Core’ and ‘Rapid’ forms, and through aligned forms by ISARIC-4C Coronavirus Clinical Characterisation Consortium in the United Kingdom [21] and the COVID-19 Critical Care Consortium [22]. Investigators from 41 countries are using Research Electronic Data Capture (REDCap, version 8.11.11, Vanderbilt University, Nashville, Tenn.) to contribute their data to a central database hosted by the University of Oxford. Additional data were submitted by investigators from Malaysia, Russia [23] and Spain, who had used alternative data collection forms and databases. This observational study required no change to clinical management and permitted enrolment in other research. The study was approved by the World Health Organization Ethics Review Committee (RPC571 and RPC572). Local ethics approval was obtained for each participating country and site according to local requirements. All investigators retained full rights to their data.
Study population
Patients of any age admitted to hospital with suspected or confirmed COVID-19 were eligible for recruitment. Some versions of the case report form included specific criteria such as fever and cough, but investigators were able to include patients with a clinical suspicion of COVID-19 even if these criteria were not met. This analysis was limited to patients who were admitted to hospital with symptomatic laboratory-confirmed COVID-19: laboratory confirmation was classified according to sites’ local diagnostic methods. We excluded asymptomatic patients who had been admitted to hospital for isolation and patients admitted for other conditions who subsequently developed COVID-19 symptoms.
Variables and measurement
Variables used in this analysis were age, sex at birth, symptoms, date of symptom onset, SARS-CoV-2 confirmation, and country of recruitment. To allow proportions to be calculated with a reliable denominator, only symptoms specified on the case report forms [15, 21, 22] were included in this analysis. The list of symptoms collected is presented with the results.
Statistical methods
Data were converted to Study Data Tabulation Model (version 1.7, Clinical Data Interchange Standards Consortium, Austin, Tex.) to allow inclusion of data submitted not using the ISARIC case report forms. We excluded patients with all symptoms recorded as missing or unknown, and those with missing age, sex or country.
Continuous variables were expressed as median with interquartile range (IQR), and categorical variables as counts with percentages. We tested for differences between female and male patients using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables. We grouped patients into ten-year age bands (with a single group for age ≥ 90 years). We plotted the most frequently reported symptoms by age group, presenting missing data as a third category. We collated symptoms according to four sets of clinical criteria. These were based on published criteria [9,10,11,12] but modified to omit symptoms with large numbers of missing data due to being added to the case report form part way through the data collection period (altered sense of taste and smell) or not being collected in all datasets (anorexia):
-
1.
Fever plus cough; or any three of fever, cough, fatigue, headache, myalgia, sore throat, rhinorrhoea, shortness of breath, nausea and vomiting, diarrhoea, and confusion;
-
2.
Cough or shortness of breath; or any two of fever, myalgia, headache, and sore throat;
-
3.
Cough or fever;
-
4.
At least one of cough, fever, and shortness of breath.
Patients with missing details of cough, fever or shortness of breath were omitted from the composite groups; patients missing details of symptoms included in the lists of criteria 1 and 2 were included, and were classified according to the non-missing symptoms. We plotted proportions of patients meeting each set of criteria by ten-year age group, with 95% confidence intervals (CI) calculated using the Clopper–Pearson method.
We used logistic regression to identify associations of age and sex with the twelve most prevalent symptoms. Age group and sex were included as fixed effects, with country as a random intercept. To display heterogeneity between countries on the same scale as the fixed effects, we calculated median odds ratios (MOR) [24]. This quantifies variation between countries by comparing odds of an outcome between randomly chosen persons in different clusters who share the same covariates [24]. MORs are defined as a comparison of the group with greater propensity to the group with lower propensity, so lie in the range 1 to infinity [24]. We plotted the MOR to show the magnitude of the effect of heterogeneity and allow comparison with the fixed effects in our data.
79% of patients were recruited in a single country (United Kingdom). As a sensitivity analysis, we repeated the analysis excluding patients from this country to see if results substantially changed. Finally, we plotted age-stratified symptom frequencies for each country with at least 250 patients.
No minimum sample size was calculated. All significance tests were two-tailed. Analyses were performed using R (version 3.6.2, R Foundation for Statistical Computing, Vienna, Austria) with packages including binom, Epi, ggplot2, lme4, sjstats, tableone, and tidyverse.
Results
Data were available for 99 623 patients. We excluded 24 336 who did not have documented SARS-CoV-2 confirmation, 3290 with missing data, and 5794 who developed COVID-19 after admission to hospital. 6094 patients were admitted to hospital with asymptomatic COVID-19, with the greatest proportion in the age band 10–20 years (46% of patients admitted in that age group). The greatest proportion of asymptomatic patients was in Malaysia (49%), where hospitalization was compulsory throughout the data collection period for people with COVID-19. We included 60 109 patients in the analysis (Fig. 1), recruited from 394 sites in 43 countries (Supplementary Table 1), in this analysis. The median age of included patients was 70 years (IQR 54–82; Table 2). 929 (1.5%) were 18 years old or younger. Age distribution of patients varied among countries, between a median of 10 years in Poland, and 73 years in the United Kingdom (Supplementary Figure 1). 34 641 (58%) patients were male.
Flow of participants in this analysis. ISARIC Core and ISARIC Rapid represent data collected internationally via two sets of case report forms; ISARIC 4C and COVID-19 CCC data were collected on separate databases using aligned case report forms; Non-REDCap data were submitted from additional sites and were collected with different case report forms. 4C, Coronavirus Clinical Characterisation Consortium; CCC, Critical Care Consortium; ISARIC, International Severe Acute Respiratory and emerging Infection Consortium; REDCap, Research Electronic Data Capture; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
The most frequently reported symptoms were fever, cough and shortness of breath (Table 3). These symptoms were each more prevalent in male patients, whereas less typical symptoms such as confusion, nausea and vomiting, diarrhoea, chest pain, headache and abdominal pain were more prevalent in female patients. The greatest sex-related difference was for nausea and vomiting, reported by 23% of female patients but only 16% of male patients. For most symptoms, the greatest prevalence was reported in adults aged between 30 and 60 years, decreasing toward extremities of age (Fig. 2). Frequency of confusion increased with age. Large numbers of patients had missing data for anorexia, severe dehydration, altered sense of taste and smell, and inability to walk, as these were not included on all case report forms. Altered sense of taste and smell, which we had excluded from the composite criteria, were experienced by only 7.4 and 6.2% respectively of patients with non-missing data.
Data on cough, fever or shortness of breath were missing for 3446 patients. The composite clinical criteria were calculated for the remaining 56 663 patients. Each set of criteria was met by a greater proportion of patients aged 30–60 years than those toward either extreme of age (Fig. 3). The criteria based on WHO’s clinical criteria [9] (fever plus cough; or any three of fever, cough, fatigue, headache, myalgia, sore throat, rhinorrhoea, shortness of breath, nausea and vomiting, diarrhoea, and confusion) were met by 40 911 (72%) patients, but only 51% of those aged 18 years and under, and 67% of those aged 70 years or over. The most sensitive criteria were at least one of cough, fever and shortness of breath, met by 52 041 (92%) participants. These criteria were met by 85% aged 18 years and under, and 90% of those aged 70 years or over. Each set of criteria were met by a greater proportion of male than female patients (Table 3).
Proportions meeting clinical criteria at hospital admission stratified by 10-year age band. Black boxes show the proportion of individuals, with error bars showing 95% confidence intervals calculated using the Clopper–Pearson method. The size of each box is inversely proportional to the variance, so larger boxes indicate greater certainty. Grey boxes with 95% confidence intervals show the proportions in the sensitivity analysis excluding patients recruited in the United Kingdom. In panel A, the three symptoms are from the list of fever, cough, fatigue, headache, myalgia, sore throat, rhinorrhoea, shortness of breath, nausea and vomiting, diarrhoea, and confusion; in panel B the two symptoms are from the list fever, myalgia, headache, and sore throat. Patients with missing data for cough, fever or shortness of breath are excluded from all four plots
For the 4622 patients whose symptoms did not meet any assessed case definitions, the most frequent symptom was confusion (47%; Table 4). This increased with age to 66% of those aged 90 years or older. Nausea and vomiting, and abdominal pain were the most common symptoms for people less than 60 years old who had not met any of the case definitions.
In the logistic regression models (Fig. 4 and 5), similar associations between age and symptoms were seen after adjustment for sex as in the unadjusted bar charts. Confusion increased with age. Nausea and vomiting, headache, abdominal pain, and sore throat were each more frequent in younger age groups, decreasing with age. Male patients had greater odds of fever, cough and shortness of breath, and lower odds of gastrointestinal symptoms of nausea and vomiting, diarrhoea, abdominal pain, chest pain, headache and sore throat. However, in each case except diarrhoea, the magnitude of the difference according to sex was much less than the effect of age. The MOR for heterogeneity between countries was greater than the relationship with sex in all symptoms (Fig. 4). It was of similar magnitude to the relationship with age for most symptoms. For each symptom, heterogeneity between countries was of a similar magnitude to the effect of age, and a greater magnitude than sex.
Odds of symptoms among patients admitted to hospital with COVID-19, stratified by age and sex. Each plot is the result of a logistic regression with a symptom as an outcome. Fixed effects of age in ten-year bands (baseline group 50–60 years) and sex are shown in black boxes with 95% confidence intervals. The size of each square is inversely proportional to the variance of the log odds ratio, so larger boxes indicate greater certainty. Clustering by country is included as a random intercept and heterogeneity is depicted by circles showing the median odds ratio
Age- and sex-specific odds of meeting clinical definitions among patients admitted to hospital with COVID-19, stratified by age and sex. Each plot is the result of a logistic regression with a composite group of symptoms as an outcome. Fixed effects of age in ten-year bands (baseline group 50–60 years) and sex are shown in black boxes with 95% confidence intervals. The size of each square is inversely proportional to the variance of the log odds ratio, so larger boxes indicate greater certainty. Clustering by country is included as a random intercept and heterogeneity is depicted by circles showing the median odds ratio. In panel A, the three symptoms are from the list of fever, cough, fatigue, headache, myalgia, sore throat, rhinorrhoea, shortness of breath, nausea and vomiting, diarrhoea, and confusion; in panel B the two symptoms are from the list fever, myalgia, headache, and sore throat. Patients with missing data for cough, fever or shortness of breath are excluded from all four plots
47 280 (79%) patients were included from the United Kingdom. Excluding these patients, the patterns of symptoms were similar to the main analysis (Supplementary Figure 2). The peak prevalence of fever, cough and shortness of breath was in 70- to 80-year olds, and fatigue increased with age. Below the age of 50 years, the clinical case definitions tended to be less sensitive in the analysis excluding the United Kingdom than in the analysis including it; above the age of 70 years each tended to be more sensitive (grey lines in Fig. 3). Within countries, the baseline prevalence of each symptom varied but patterns within countries were broadly similar to the overall results (Supplementary Figures 3–14).
Discussion
The ISARIC prospective multinational cohort study is the largest cohort of patients admitted to hospital with COVID-19 to date. In this report, we confirmed a relationship between patients’ symptoms and their age and sex. The ‘typical’ COVID-19 symptoms occur most frequently in adults aged 30–60 years. Commonly used case definitions can miss up to half of children and a third of adults over 70 years who are admitted to hospital with COVID-19.
Our results support the findings of smaller cohort studies that atypical symptoms are more common in older adults [26], and correlate with similar findings of atypical presentations for pneumonia, bacteraemia and coronary artery disease [27, 28]. A lower prevalence of symptoms in children and young people has previously been suggested [29, 30], but this is the first large international cohort to collect data prospectively from both adults and children.
Separate analyses of the ISARIC-4C data have identified fever, cough and shortness of breath as frequently co-occurring clusters of symptoms [31, 32]. In children, the next most frequent cluster consisted of systemic, enteric and mucocutaneous symptoms [31]. For adults, other clusters consisted of non-specific viral symptoms, gastrointestinal symptoms, upper respiratory symptoms, neurological symptoms, and symptoms of bronchospasm [32]. Those data were included in this global dataset so the results of these analyses are not independent of our results.
We found that differences in symptoms by sex were statistically significant but generally of smaller magnitude. Typical symptoms of fever, cough and shortness of breath were more common in men than in women; all other symptoms were equal or more common in female patients. A cohort of non-hospitalized patients with COVID-19 in Poland found greater differences in symptoms of lack of appetite (55% of women, 36% of men) and taste disorder (53% women, 40% men) [33]. We are unable to determine from our data whether these differences reflect differences in health-seeking behaviour between men and women, or physiological differences in their response to the infection. Elaboration of this difference should be a goal of future research.
Our results suggest considerable heterogeneity among countries. We have not attempted to elicit reasons for heterogeneity. Potential reasons include cultural idiosyncrasies in reporting symptoms, and hospitals’ criteria for admission and testing. It might also reflect differences in local patient recruitment. Researchers in some countries may be unwilling to recruit confused patients due to requirements for consent, whereas in others, the requirement for consent has been waived or could be obtained from a proxy. We explored the effect of using the country of recruitment as a random effect in regression models and by repeating the analysis excluding the largest country. Each analysis suggested an underlying pattern of lower frequencies of typical symptoms in children and older adults. Therefore, although the prevalence of each symptom reported in this study may not apply to all settings, we have evidence to support the possibility of age-dependent differences internationally.
The size of this cohort is a strength. To our knowledge, it is the largest cohort of hospitalized COVID-19 patients in the world. However, the study has several limitations. First, almost 80% of patients were recruited in a single country. Moreover, less than 1% of patients were recruited from low- or lower–middle-income countries. Second, the cohort overwhelmingly includes older adults, with only 1.8% of the cohort aged 18 years or younger. Third, our analysis includes only patients who were hospitalized with COVID-19 and who had a laboratory-confirmed diagnosis. This patient population is more likely to be severely unwell and more likely to exhibit symptoms typically associated with COVID-19 than people who were managed in the community or whose disease has not been recognized. Accordingly, the reporting of ‘typical’ COVID-19 symptoms in this cohort is likely to be an overestimate of the population prevalence. Symptoms are subjective and cannot be externally verified. Some differences for children may reflect that symptoms could only be recorded if a caregiver recognised the symptom or the child had the appropriate vocabulary to describe it. Similarly, some symptoms may be under-reported in elderly patients if there are difficulties in communication, for example due to delirium. As such, the generalizability of estimates of our symptom prevalence is limited. Similarly, there is a shortage of studies conducted outside of high-income countries: a recent scoping review of clinical characteristics of COVID-19 identified no large cohorts in non-high-income countries except China [8].
The absence of a control group of patients without COVID-19 in this dataset prevented estimation of specificity or positive and negative predictive values. We are therefore prevented from advocating changes to clinical case definitions, as such decisions inevitably require a balance of false-positive and false-negative rates. However, given the prevalence of atypical symptoms in our cohort, we can confidently suggest that reliance on clinical case definitions may result in missing cases of COVID-19, especially among children and older adults. Non-healthcare professionals making decisions regarding isolation may be especially vulnerable to missing cases of COVID-19 by adhering to a clinical case definition too strictly.
The reported prevalence of COVID-19 may also rely on a strict interpretation of case definitions. In settings where comprehensive contact tracing is planned, or there is easy access to microbiological testing, a highly sensitive case definition is desirable. However, where decisions are based on clinical diagnoses, it is important to recognize other pathogens that can cause similar constellations of symptoms. The addition of symptoms such as confusion or gastrointestinal symptoms to the COVID-19 case definition could increase sensitivity, but at the cost of reduced specificity. Changes in the senses of taste and smell have recently been added to case definitions. Our data suggest that these criteria would detect only a small proportion of patients admitted to hospital with COVID-19 who were omitted by other definitions.
These results highlight the need to consider COVID-19 even if individuals do not display typical symptoms of the disease. This is especially the case in children and older adults. Given that our results are likely to overestimate the sensitivity of the clinical criteria currently used to identify patients for testing, our results suggest a lower limit to the proportion of people in the community with COVID-19 who would not be identified. Addition of confusion as a symptom would increase the sensitivity of case definitions for older adults; and inclusion of nausea and vomiting or abdominal pain would increase sensitivity for children and young adults. The high proportion of asymptomatic patients identified in patients aged 10–20 years suggests that universal screening in these ages could be beneficial when there is widespread community circulation of COVID-19. Ongoing data collection outside high-income countries is needed to establish whether alternative case definitions are needed in different settings. Work is also ongoing to determine whether some constellations of symptoms are associated with better or poorer outcomes than others.
Availability of data and materials
We welcome applications for UK data through the ISARIC 4C Independent Data and Material Access Committee (https://isaric4c.net). Requests for access to non-UK data can be sent to covid19@iddo.org.
References
Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7(7):Cd013665. https://doi.org/10.1002/14651858.Cd013665.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/s0140-6736(20)30183-5.
Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020;55(6):2000562. https://doi.org/10.1183/13993003.00562-2020.
Wang G, Chen W, Jin X, Chen YP. Description of COVID-19 cases along with the measures taken on prevention and control in Zhejiang. China J Med Virol. 2020. https://doi.org/10.1002/jmv.25906.
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71(15):889–90. https://doi.org/10.1093/cid/ciaa330.
Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806–13. https://doi.org/10.1002/alr.22579.
Manoharan L, Cattrall JWS, Harris C, Newell K, Thomson B, Pritchard MG, et al. Early clinical characteristics of Covid-19: scoping review. MedRxiv. 2020. https://doi.org/10.1101/2020.07.31.20165738.
World Health Organization. WHO COVID-19: Case definitions [WHO/2019-nCoV/Surveillance_Case_Definition/2020.1]. Geneva, Switzerland: World Health Organization, 2020
Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) 2020 interim case definition Atlanta, GA: Centers for Disease Control and Prevention; 2020 [cited 2020 9 November]. https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/.
Public Health England. COVID19: investigation and initial clinical management of possible cases: Public Health England; 2020 [cited 2020 9 November]. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection.
European Centre for Disease Prevention and Control. Case definition for coronavirus disease 2019 (COVID-19), as of 29 May 2020: European Centre for Disease Prevention and Control; 2020 [cited 2020 9 November]. https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition.
ISARIC clinical characterisation group. Global outbreak research: harmony not hegemony. Lancet Infect Dis. 2020;20(7):770–2.. doi: https://doi.org/10.1016/s1473-3099(20)30440-0.
World Health Organization (2020) International Severe Acute Respiratory and Emerging Infection Consortium. ISARIC/WHO Clinical Characterisation Protocol for Severe Emerging Infections, version 3.2. ISARIC, 2020
COVID-19 CRF (2020) International severe acute respiratory and emerging infection consortium [21 August 2020]. https://isaric.org/research/covid-19-clinical-research-resources/covid-19-crf/
Dunning JW, Merson L, Rohde GGU, Gao Z, Semple MG, Tran D, et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14(1):8–9. https://doi.org/10.1016/s1473-3099(13)70327-x.
Pritchard M, Dankwa EA, Hall M, Baillie JK, Carson G, Docherty A, et al. ISARIC COVID-19 clinical data report. MedRxiv. 2020. https://doi.org/10.1101/2020.07.17.20155218.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. https://doi.org/10.1016/s2213-2600(20)30079-5.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/s0140-6736(20)30566-3.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. https://doi.org/10.1136/bmj.m1985.
ISARIC 4C (2020) ISARIC 4C protocols 2020 [26 August 2020]. https://isaric4c.net/protocols/.
COVID-19 Critical Care Consortium (2020) COVID-19 Critical Care Consortium Trial Documents 2020 [26 August 2020]. https://www.elso.org/COVID19/ECMOCARD.aspx.
Munblit D, Nekliudov NA, Bugaeva P, Blyuss O, Kislova M, Listovskaya E, et al. StopCOVID cohort: an observational study of 3,480 patients admitted to the Sechenov University hospital network in Moscow city for suspected COVID-19 infection. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa1535.
Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161(1):81–8. https://doi.org/10.1093/aje/kwi017.
World Bank (2020) World Bank country and lending groups 2020. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
Lauretani F, Ravazzoni G, Roberti MF, Longobucco Y, Adorni E, Grossi M, et al. Assessment and treatment of older individuals with COVID 19 multi-system disease: clinical and ethical implications. Acta Biomed. 2020;91(2):150–68. https://doi.org/10.23750/abm.v91i2.9629.
Jung YJ, Yoon JL, Kim HS, Lee AY, Kim MY, Cho JJ. Atypical clinical presentation of geriatric syndrome in elderly patients with pneumonia or coronary artery disease. Ann Geriatr Med Res. 2018;21(4):158–63.
Wester AL, Dunlop O, Melby KK, Dahle UR, Wyller TB. Age-related differences in symptoms, diagnosis and prognosis of bacteremia. BMC Infect Dis. 2013. https://doi.org/10.1186/1471-2334-13-346.
Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–5. https://doi.org/10.1056/NEJMc2005073.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95. https://doi.org/10.1111/apa.15270.
Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. https://doi.org/10.1136/bmj.m3249%JBMJ.
Millar JE, Neyton L, Seth S, Dunning J, Merson L, Murthy S, et al. Robust, reproducible clinical patterns in hospitalised patients with COVID-19. MedRxiv. 2020. https://doi.org/10.1101/2020.08.14.20168088.
Sierpiński R, Pinkas J, Jankowski M, Zgliczyński WS, Wierzba W, Gujski M, et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID-19). Pol Arch Intern Med. 2020;130(6):501–5. https://doi.org/10.20452/pamw.15414.
Acknowledgements
We are extremely grateful to the frontline clinical and research staff and volunteers, who collected this data in challenging circumstances, and the generosity of the patients and their families for their individual contributions in these difficult times. This work uses data provided by patients and collected by the health institutions and authorities in each country. In the UK, this study involves the National Health Service as part of their care and support #DataSavesLives. We also acknowledge the support of Jeremy J Farrar, Nahoko Shindo, Devika Dixit, Nipunie Rajapakse, Andrew Davison, Lyndsey Castle, Martha Buckley, Debbie Malden, Katherine Newell, Kwame O’Neill, Emmanuelle Denis, Claire Petersen, Scott Mullaney, Sue MacFarlane, Nicole Maziere, Julien Martinez, Oslem Dincarslan, Annette Lake and the Irish Critical Care Trials Group. We appreciate the strong collaboration of the WHO Clinical Data Platform Team, including Silvia Bertagnolio, Soe Soe Thwin, and Janet Diaz.
ISARIC Clinical Characterisation Group:
Sheryl Ann Abdukahil, Ryuzo Abe, Laurent Abel, Lara Absil, Andrew Acker, Shingo Adachi, Elisabeth Adam, Diana Adrião, Kate Ainscough, Ali Ait Hssain, Younes Ait Tamlihat, Takako Akimoto, Tala Al-Dabbous, Abdulrahman Al-Fares, Eman Al Qasim, Razi Alalqam, Beatrice Alex, Kévin Alexandre, Huda Alfoudri, Kazali Enagnon Alidjnou, Jeffrey Aliudin, Clotilde Allavena, Nathalie Allou, João Alves, Rita Alves, Maria Amaral, Heidi Ammerlaan, Phoebe Ampaw, Roberto Andini, Claire Andrejak, Andrea Angheben, François Angoulvant, Séverine Ansart, Massimo Antonelli, Carlos Alexandre Antunes De Brito, Yaseen Arabi, Irene Aragao, Antonio Arcadipane, Lukas Arenz, Jean-Benoît Arlet, Christel Arnold-Day, Lovkesh Arora, Elise Artaud-Macari, Angel Asensio, Jean Baptiste Assie, Anika Atique, Johann Auchabie, Hugues Aumaitre, Laurène Azemar, Cécile Azoulay, Benjamin Bach, Delphine Bachelet, J. Kenneth Baillie, Erica Bak, Agamemnon Bakakos, Firouzé Banisadr, Renata Barbalho, Wendy S. Barclay, Michaela Barnikel, Audrey Barrelet, Cleide Barrigoto, Romain Basmaci, Diego Fernando Bautista Rincon, Alexandra Bedossa, Sylvie Behilill, Aleksandr Beljantsev, David Bellemare, Anna Beltrame, Marine Beluze, Nicolas Benech, Dehbia Benkerrou, Suzanne Bennett, LuÍs Bento, Jan-Erik Berdal, Delphine Bergeaud, Lorenzo Bertolino, Simon Bessis, Sybille Bevilcaqua, Krishna Bhavsar, Felwa Bin Humaid, François Bissuel, Patrick Biston, Laurent Bitker, Pablo Blanco-Schweizer, Mathieu Blot, Filomena Boccia, Debby Bogaert, François Bompart, Gareth Booth, Diogo Borges, Raphaël Borie, Jeannet Bos, Hans Martin Bosse, Elisabeth Botelho-Nevers, Lila Bouadma, Olivier Bouchaud, Sabelline Bouchez, Dounia Bouhmani, Damien Bouhour, Kévin Bouiller, Laurence Bouillet, Camille Bouisse, Anne-Sophie Boureau, Maude Bouscambert, Jason Bouziotis, Bianca Boxma, Marielle Boyer-Besseyre, Maria Boylan, Cynthia Braga, Timo Brandenburger, Luca Brazzi, Dorothy Breen, Patrick Breen, Kathy Brickell, Nicolas Brozzi, Nina Buchtele, Christian Buesaquillo, Polina Bugaeva, Marielle Buisson, Erlina Burhan, Ingrid G. Bustos, Denis Butnaru, Sheila Cárcel, André Cabie, Susana Cabral, Eder Caceres, Mia Callahan, Kate Calligy, Jose Andres Calvache, João Camões, Valentine Campana, Paul Campbell, Cecilia Canepa, Mireia Cantero, Pauline Caraux-Paz, Filipa Cardoso, Filipe Cardoso, Sofia Cardoso, Simone Carelli, Nicolas Carlier, Gayle Carney, Chloe Carpenter, Marie-Christine Carret, François Martin Carrier, Gail Carson, Maire-Laure Casanova, Mariana Cascão, José Casimiro, Bailey Cassandra, Silvia Castañeda, Nidyanara Castanheira, Guylaine Castor-Alexandre, Henry Castrillón, Ivo Castro, Ana Catarino, François-Xavier Catherine, Roberta Cavalin, Giulio Giovanni Cavalli, Alexandros Cavayas, Adrian Ceccato, Minerva Cervantes-Gonzalez, Anissa Chair, Catherine Chakveatze, Adrienne Chan, Meera Chand, Julie Chas, Camille Chassin, Anjellica Chen, Yih-Sharng Chen, Matthew Pellan Cheng, Antoine Cheret, Thibault Chiarabini, Julian Chica, Catherine Chirouze, Davide Chiumello, Hwa Jin Cho, Sung Min Cho, Bernard Cholley, Jose Pedro Cidade, Jose Miguel Cisneros Herreros, Barbara Wanjiru Citarella, Anna Ciullo, Jennifer Clarke, Sara Clohisey, Cassidy Codan, Caitriona Cody, Alexandra Coelho, Gwenhaël Colin, Michael Collins, Sebastiano Maria Colombo, Pamela Combs, JP Connelly, Marie Connor, Anne Conrad, Sofía Contreras, Graham S. Cooke, Mary Copland, Hugues Cordel, Amanda Corley, Sarah Cormican, Sabine Cornelis, Arianne Joy Corpuz, Grégory Corvaisier, Camille Couffignal, Sandrine Couffin-Cadiergues, Roxane Courtois, Charles Crepy D’Orleans, Sabine Croonen, Gloria Crowl, Jonathan Crump, Claudina Cruz, Marc Csete, Alberto Cucino, Caroline Cullen, Matthew Cummings, Gerard Curley, Elodie Curlier, Paula Custodio, Frédérick D’Aragon, Ana Da Silva Filipe, Charlene Da Silveira, Eric D’Ortenzio, Al-Awwab Dabaliz, Andrew B. Dagens, Heidi Dalton, Jo Dalton, Nick Daneman, Emmanuelle A. Dankwa, Jorge Dantas, Nathalie De Castro, Diego De Mendoza, Rafael Freitas De Oliveira França, Rosanna De Rosa, Thushan De Silva, Peter De Vries, David Dean, Marie-Pierre Debray, William Dechert, Lauren Deconninck, Romain Decours, Isabelle Delacroix, Karen Delavigne, Ionna Deligiannis, Andrea Dell’amore, Pierre Delobel, Elisa Demonchy, Emmanuelle Denis, Dominique Deplanque, Dominique Deplanque, Pieter Depuydt, Mehul Desai, Diane Descamps, Mathilde Desvallée, Santi Rahayu Dewayanti, Alpha Diallo, Sylvain Diamantis, André Dias, Juan Jose Diaz Diaz, Rodrigo Diaz, Kévin Didier, Jean-Luc Diehl, Wim Dieperink, Jérôme Dimet, Vincent Dinot, Alphonsine Diouf, Yael Dishon, Félix Djossou, Annemarie B. Docherty, Andy Dong, Christl A. Donnelly, Maria Donnelly, Chloe Donohue, Céline Dorival, James Joshua Douglas, Renee Douma, Nathalie Dournon, Triona Downer, Mark Downing, Tom Drake, Vincent Dubee, François Dubos, Alexandra Ducancelle, Susanne Dudman, Jake Dunning, Emanuele Durante Mangoni, Silvia Duranti, Lucian Durham III, Bertrand Dussol, Xavier Duval, Anne Margarita Dyrhol-Riise, Carla Eira, José Ernesto Vidal, Mohammed El Sanharawi, Subbarao Elapavaluru, Brigitte Elharrar, Natalie Elkheir, Jacobien Ellerbroek, Rachael Ellis, Philippine Eloy, Tarek Elshazly, Isabelle Enderle, Ilka Engelmann, Vincent Enouf, Olivier Epaulard, Mariano Esperatti, Hélène Esperou, Marina Esposito-Farese, João Estevão, Manuel Etienne, Manuel Etienne, Nadia Ettalhaoui, Anna Greti Everding, Mirjam Evers, Isabelle Fabre, Amna Faheem, Arabella Fahy, Cameron J. Fairfield, Pedro Faria, Nataly Farshait, Arie Zainul Fatoni, Karine Faure, Mohamed Fayed, Niamh Feely, Jorge Fernandes, Marília Fernandes, Susana Fernandes, Joana Ferrão, Eglantine Ferrand Devouge, Mário Ferraz, Benigno Ferreira, Ricard Ferrer-Roca, Claudia Figueiredo-Mello, Clara Flateau, Tom Fletcher, Letizia Lucia Florio, Claire Foley, Victor Fomin, Claudio Duarte Fonseca, Tatiana Fonseca, Patricia Fontela, Simon Forsyth, Giuseppe Foti, Erwan Fourn, Rob Fowler, Diego Franch-Llasat, Christophe Fraser, John Fraser, Marcela Vieira Freire, Ana Freitas Ribeiro, Caren Friedrich, Stéphane Fry, Nora Fuentes, Masahiro Fukuda, Joan Gómez-Junyent, Valérie Gaborieau, Benoît Gachet, Rostane Gaci, Massimo Gagliardi, Jean-Charles Gagnard, Amandine Gagneux-Brunon, Sérgio Gaião, Phil Gallagher, Elena Gallego Curto, Carrol Gamble, Arthur Garan, Esteban Garcia-Gallo, Rebekha Garcia, Denis Garot, Valérie Garrait, Nathalie Gault, Aisling Gavin, Alexandre Gaymard, Johannes Gebauer, Louis Gerbaud Morlaes, Nuno Germano, Jade Ghosn, Marco Giani, Carlo Giaquinto, Jess Gibson, Tristan Gigante, Morgane Gilg, Guillermo Giordano, Michelle Girvan, Valérie Gissot, Gezy Giwangkancana, Daniel Glikman, Petr Glybochko, Eric Gnall, Geraldine Goco, François Goehringer, Siri Goepel, Jean-Christophe Goffard, Jonathan Golob, Isabelle Gorenne, Cécile Goujard, Tiphaine Goulenok, Margarite Grable, Edward Wilson Grandin, Pascal Granier, Giacomo Grasselli, Christopher A. Green, William Greenhalf, Segolène Greffe, Domenico Luca Grieco, Matthew Griffee, Fiona Griffiths, Ioana Grigoras, Albert Groenendijk, Anja Grosse Lordemann, Heidi Gruner, Yusing Gu, Jérémie Guedj, Dewi Guellec, Anne-Marie Guerguerian, Daniela Guerreiro, Romain Guery, Anne Guillaumot, Laurent Guilleminault, Thomas Guimard, Daniel Haber, Sheeba Hakak, Matthew Hall, Sophie Halpin, Ansley Hamer, Rebecca Hamidfar, Terese Hammond, Hayley Hardwick, Kristen Harley, Ewen M. Harrison, Janet Harrison, Leanne Hays, Jan Heerman, Lars Heggelund, Ross Hendry, Martina Hennessy, Aquiles Henriquez-Trujillo, Maxime Hentzien, Jaime Hernandez-Montfort, Astarini Hidayah, Dawn Higgins, Eibhilin Higgins, Samuel Hinton, Ana Hipólito -Reis, Hiroaki Hiraiwa, Julian A. Hiscox, Antonia Ying Wai Ho, Alexandre Hoctin, Isabelle Hoffmann, Oscar Hoiting, Rebecca Holt, Jan Cato Holter, Peter Horby, Juan Pablo Horcajada, Koji Hoshino, Kota Hoshino, Catherine L. Hough, Jimmy Ming-Yang Hsu, Jean-Sébastien Hulot, Samreen Ijaz, Hajnal-Gabriela Illes, Hugo Inácio, Carmen Infante Dominguez, Elias Iosifidis, Lacey Irvine, Sarah Isgett, Tiago Isidoro, Margaux Isnard, Junji Itai, Daniel Ivulich, Salma Jaafoura, Julien Jabot, Clare Jackson, Nina Jamieson, Stéphane Jaureguiberry, Jeffrey Javidfar, Zabbe Jean-Benoît, Florence Jego, Synne Jenum, Ruth Jimbo Sotomayor, Ruth Noemí Jorge GarcÍa, Cédric Joseph, Mark Joseph, Philippe Jouvet, Hanna Jung, Ouifiya Kafif, Florentia Kaguelidou, Sabina Kali, Smaragdi Kalomoiri, Darshana Hewa Kandamby, Chris Kandel, Ravi Kant, Christiana Kartsonaki, Daisuke Kasugai, Kevin Katz, Simreen Kaur Johal, Sean Keating, Andrea Kelly, Sadie Kelly, Lisa Kennedy, Kalynn Kennon, Younes Kerroumi, Evelyne Kestelyn, Imrana Khalid, Antoine Khalil, Coralie Khan, Irfan Khan, Michelle E Kho, Saye Khoo, Yuri Kida, Peter Kiiza, Anders Benjamin Kildal, Antoine Kimmoun, Detlef Kindgen-Milles, Nobuya Kitamura, Paul Klenerman, Gry Kloumann Bekken, Stephen Knight, Robin Kobbe, Malte Kohns Vasconcelos, Volkan Korten, Caroline Kosgei, Karolina Krawczyk, Pavan Kumar Vecham, Deepali Kumar, Ethan Kurtzman, Demetrios Kutsogiannis, Konstantinos Kyriakoulis, Erwan L'her, Marie Lachatre, Marie Lacoste, John G Laffey, Marie Lagrange, Fabrice Laine, Marc Lambert, François Lamontagne, Marie Langelot-Richard, Eka Yudha Lantang, Marina Lanza, Cédric Laouénan, Samira Laribi, Delphine Lariviere, Odile Launay, Yoan Lavie-Badie, Andrew Law, Clément Le Bihan, Cyril Le Bris, Eve Le Coustumier, Georges Le Falher, Sylvie Le Gac, Quentin Le Hingrat, Marion Le Maréchal, Soizic Le Mestre, Vincent Le Moing, Hervé Le Nagard, Paul Le Turnier, Rafael León, Minh Le, Marta Leal Santos, Ema Leal, James Lee, Su Hwan Lee, Todd Lee, Gary Leeming, Bénédicte Lefebvre, Laurent Lefebvre, Benjamin Lefevre, François Lellouche, Adrien Lemaignen, Véronique Lemee, Gretchen Lemmink, Michela Leone, Quentin Lepiller, François-Xavier Lescure, Olivier Lesens, Mathieu Lesouhaitier, Claire Levy-Marchal, Bruno Levy, Yves Levy, Gianluigi Li Bassi, Janet Liang, Wei Shen Lim, Bruno Lina, Andreas Lind, Guillaume Lingas, Sylvie Lion-Daolio, Keibun Liu, Antonio Loforte, Navy Lolong, Diogo Lopes, Dalia Lopez-Colon, Paul Loubet, Jean Christophe Lucet, Carlos M. Luna, Olguta Lungu, Liem Luong, Dominique Luton, Ruth Lyons, Fredrik Müller, Karl Erik Müller, Olavi Maasikas, Sarah Macdonald, Moïse Machado, Gabrielle Macheda, Juan Macias Sanchez, Jai Madhok, Rafael Mahieu, Sophie Mahy, Lars Siegfrid Maier, Mylène Maillet, Thomas Maitre, Maximilian Malfertheiner, Nadia Malik, Fernando Maltez, Denis Malvy, Marina Mambert, Victoria Manda, Jose M. Mandei, Edmund Manning, Aldric Manuel, Ceila Maria Sant`Ana Malaque, Flávio Marino, Carolline De Araújo Mariz, Charbel Maroun Eid, Ana Marques, Catherine Marquis, Brian Marsh, Laura Marsh, John Marshall, Celina Turchi Martelli, Guillaume Martin-Blondel, Ignacio Martin-Loeches, Alejandro Martin-Quiros, Dori-Ann Martin, Emily Martin, Martin Martinot, Caroline Martins Rego, Ana Martins, João Martins, Gennaro Martucci, Eva Miranda Marwali, Juan Fernado Masa Jimenez, David Maslove, Sabina Mason, Moshe Matan, Daniel Mathieu, Mathieu Mattei, Romans Matulevics, Laurence Maulin, Natalie Mc Evoy, Aine McCarthy, Anne McCarthy, Colin McCloskey, Rachael McConnochie, Sherry McDermott, Sarah McDonald, Samuel McElwee, Natalie McEvoy, Allison McGeer, Niki McGuinness, Kenneth A. McLean, Bairbre McNicholas, Edel Meaney, Cécile Mear-Passard, Maggie Mechlin, Ferruccio Mele, Kusum Menon, France Mentré, Alexander J. Mentzer, Noémie Mercier, Antoine Merckx, Blake Mergler, Laura Merson, António Mesquita, Agnès Meybeck, Alison M. Meynert, Vanina Meyssonnier, Amina Meziane, Medhi Mezidi, Céline Michelanglei, Vladislav Mihnovitš, Hugo Miranda Maldonado, Mary Mone, Asma Moin, David Molina, Elena Molinos, Agostinho Monteiro, Claudia Montes, Giorgia Montrucchio, Sarah Moore, Shona C. Moore, Lina Morales-Cely, Lucia Moro, Diego Rolando Morocho Tutillo, Ana Motos, Hugo Mouquet, Clara Mouton Perrot, Julien Moyet, Jimmy Mullaert, Daniel Munblit, Derek Murphy, Marlène Murris, Dimitra Melia Myrodia, Yohan N’guyen, Nadège Neant, Holger Neb, Nikita A Nekliudov, Raul Neto, Emily Neumann, Bernardo Neves, Pauline Yeung Ng, Wing Yiu Ng, Orna Ni Choileain, Alistair Nichol, Stephanie Nonas, Marion Noret, Lisa Norman, Alessandra Notari, Mahdad Noursadeghi, Karolina Nowicka, Saad Nseir, Jose I Nunez, Elsa Nyamankolly, Max O’Donnell, Katie O’Hearn, Conar O’Neil, Giovanna Occhipinti, Tawnya Ogston, Takayuki Ogura, Tak-Hyuk Oh, Shinichiro Ohshimo, Budha Charan Singh Oinam, Ana Pinho Oliveira, João Oliveira, Piero Olliaro, David S. Y. Ong, Wilna Oosthuyzen, Peter J.M. Openshaw, Claudia Milena Orozco-Chamorro, Andrés Orquera, Javier Osatnik, Nadia Ouamara, Rachida Ouissa, Clark Owyang, Eric Oziol, Diana Póvoas, Maïder Pagadoy, Justine Pages, Mario Palacios, Massimo Palmarini, Giovanna Panarello, Prasan Kumar Panda, Mauro Panigada, Nathalie Pansu, Aurélie Papadopoulos, Briseida Parra, Jérémie Pasquier, Fabian Patauner, Luís Patrão, Christelle Paul, Mical Paul, Jorge Paulos, William A. Paxton, Jean-François Payen, India Pearse, Giles J Peek, Florent Peelman, Nathan Peiffer-Smadja, Vincent Peigne, Mare Pejkovska, Ithan D. Peltan, Rui Pereira, Daniel Perez, Thomas Perpoint, Antonio Pesenti, Lenka Petroušová, Ventzislava Petrov-Sanchez, Gilles Peytavin, Scott Pharand, Michael Piagnerelli, Walter Picard, Olivier Picone, Marie Piel-Julian, Carola Pierobon, Carlos Pimentel, Lionel Piroth, Riinu Pius, Simone Piva, Laurent Plantier, Daniel Plotkin, Julien Poissy, Maria Pokorska-Spiewak, Sergio Poli, Georgios Pollakis, Jolanta Popielska, Douwe F. Postma, Pedro Povoa, Jeff Powis, Sofia Prapa, Christian Prebensen, Jean-Charles Preiser, Vincent Prestre, Nicholas Price, Anton Prinssen, Mark G. Pritchard, Lúcia Proença, Oriane Puéchal, Gregory Purcell, Luisa Quesada, Else Quist-Paulsen, Mohammed Quraishi, Indrek Rätsep, Bernhard Rössler, Christian Rabaud, Marie Rafiq, Gabrielle Ragazzo, Fernando Rainieri, Nagarajan Ramakrishnan, Kollengode Ramanathan, Blandine Rammaert, Christophe Rapp, Menaldi Rasmin, Cornelius Rau, Stanislas Rebaudet, Sarah Redl, Brenda Reeve, Liadain Reid, Renato Reis, Jonathan Remppis, Martine Remy, Hanna Renk, Liliana Resende, Anne-Sophie Resseguier, Matthieu Revest, Oleksa Rewa, Luis Felipe Reyes, David Richardson, Denise Richardson, Laurent Richier, Jordi Riera, Ana Lúcia Rios, Asgar Rishu, Patrick Rispal, Karine Risso, Maria Angelica Rivera Nuñez, Nicholas Rizer, André Roberto, Stephanie Roberts, David L. Robertson, Olivier Robineau, Ferran Roche-Campo, Paola Rodari, Simão Rodeia, Julia Rodriguez Abreu, Emmanuel Roilides, Amanda Rojek, Juliette Romaru, Roberto Roncon-Albuquerque Jr, Mélanie Roriz, Manuel Rosa-Calatrava, Michael Rose, Dorothea Rosenberger, Andrea Rossanese, Bénédicte Rossignol, Patrick Rossignol, Carine Roy, Benoît Roze, Clark D. Russell, Steffi Ryckaert, Aleksander Rygh Holten, Xavier Sánchez Choez, Isabela Saba, Musharaf Sadat, Nadia Saidani, Leonardo Salazar, Gabriele Sales, Stéphane Sallaberry, Hélène Salvator, Angel Sanchez-Miralles, Olivier Sanchez, Vanessa Sancho-Shimizu, Gyan Sandhu, Oana Sandulescu, Marlene Santos, Shirley Sarfo-Mensah, Benjamine Sarton, Egle Saviciute, Parthena Savvidou, Joshua Scarsbrook, Tjard Schermer, Arnaud Scherpereel, Marion Schneider, Stephan Schroll, Michael Schwameis, James Scott-Brown, Janet T. Scott, Nicholas Sedillot, Tamara Seitz, Caroline Semaille, Malcolm G. Semple, Eric Senneville, Filipa Sequeira, Tânia Sequeira, Ellen Shadowitz, Mohammad Shamsah, Pratima Sharma, Catherine A. Shaw, Victoria Shaw, Nisreen Shiban, Nobuaki Shime, Hiroaki Shimizu, Keiki Shimizu, Sally Shrapnel, Hoi Ping Shum, Nassima Si Mohammed, Louise Sigfrid, Catarina Silva, Maria Joao Silva, Wai Ching Sin, Vegard Skogen, Sue Smith, Benjamin Smood, Michelle Smyth, Morgane Snacken, Dominic So, Monserrat Solis, Joshua Solomon, Tom Solomon, Emily Somers, Agnès Sommet, Myung Jin Song, Rima Song, Tae Song, Michael Sonntagbauer, Edouard Soum, Maria Sousa Uva, Marta Sousa, Vicente Souza-Dantas, Alexandra Sperry, Shiranee Sriskandan, Thomas Staudinger, Stephanie-Susanne Stecher, Ymkje Stienstra, Birgitte Stiksrud, Adrian Streinu-Cercel, Anca Streinu-Cercel, Samantha Strudwick, Ami Stuart, David Stuart, Asfia Sultana, Charlotte Summers, Magdalena Surovcová Andrey A Svistunov, Konstantinos Syrigos, Jaques Sztajnbok, Konstanty Szuldrzynski, François Téoulé, Shirin Tabrizi, Lysa Tagherset, Ewa Talarek, Sara Taleb, Jelmer Talsma, Le Van Tan, Hiroyuki Tanaka, Taku Tanaka, Hayato Taniguchi, Coralie Tardivon, Pierre Tattevin, M Azhari Taufik, Richard S. Tedder, João Teixeira, Marie-Capucine Tellier, Pleun Terpstra, Olivier Terrier, Nicolas Terzi, Hubert Tessier-Grenier, Vincent Thibault, Simon-Djamel Thiberville, Benoît Thill, A.A. Roger Thompson, Shaun Thompson, David Thomson, Emma C. Thomson, Duong Bich Thuy, Ryan S. Thwaites, Peter S. Timashev, Jean-François Timsit, Bharath Kumar Tirupakuzhi Vijayaraghavan, Maria Toki, Kristian Tonby, Rosario Maria Torres Santos-Olmo, Antoni Torres, Margarida Torres, Théo Trioux, Huynh Trung Trieu, Cécile Tromeur, Ioannis Trontzas, Jonathan Troost, Tiffany Trouillon, Christelle Tual, Sarah Tubiana, Helen Tuite, Lance C.W. Turtle, Pawel Twardowski, Makoto Uchiyama, Roman Ullrich, Alberto Uribe, Asad Usman, Luís Val-Flores, Stijn Van De Velde, Marcel Van Den Berge, Machteld Van Der Feltz, Nicky Van Der Vekens, Peter Van Der Voort, Sylvie Van Der Werf, Marlice Van Dyk, Laura Van Gulik, Jarne Van Hattem, Steven Van Lelyveld, Carolien Van Netten, Noémie Vanel, Henk Vanoverschelde, Charline Vauchy, Aurélie Veislinger, Jorge Velazco, Sara Ventura, Annelies Verbon, César Vieira, Joy Ann Villanueva, Judit Villar, Pierre-Marc Villeneuve, Andrea Villoldo, Nguyen Van Vinh Chau, Benoit Visseaux, Hannah Visser, Aapeli Vuorinen, Fanny Vuotto, Chih-Hsien Wang, Jia Wei, Katharina Weil, Sanne Wesselius, Murray Wham, Bryan Whelan, Nicole White, Aurélie Wiedemann, Keith Wille, Evert-Jan Wils, Ioannis Xynogalas, Jacky Y Suen, Sophie Yacoub, Masaki Yamazaki, Yazdan Yazdanpanah, Cécile Yelnik, Stephanie Yerkovich, Toshiki Yokoyama, Hodane Yonis, Paul Young, Saptadi Yuliarto, Marion Zabbe, Kai Zacharowski, Maram Zahran, Maria Zambon, Alberto Zanella, Konrad Zawadka, Hiba Zayyad, Alexander Zoufaly, David Zucman; The PETAL Network Investigators, The Western Australian Covid 19 Research Response.
Funding
This work was supported by the Department for International Development and Wellcome Trust [215091/Z/18/Z]; the Bill and Melinda Gates Foundation [OPP1209135]. Country-specific support was provided by the Canadian Institutes of Health Research Coronavirus Rapid Research Funding Opportunity [OV2170359]; the Health Research Board Ireland [CTN Award 2014-012]; National Institute for Health Research (NIHR) [award CO-CIN-01]; the Medical Research Council (MRC) [grant MC_PC_19059]; the NIHR Health Protection Research Unit (HPRU) in Emerging and Zoonotic Infections at University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford [award 200907]; NIHR HPRU in Respiratory Infections at Imperial College London with PHE [award 200927]; Liverpool Experimental Cancer Medicine Centre [grant reference C18616/A25153]; NIHR Biomedical Research Centre at Imperial College London [IS-BRC-1215-20013]; EU Platform for European Preparedness Against (Re-)emerging Epidemics (PREPARE) [FP7 project 602525]; National Institutes of Health (NIH) [UL1TR002240]; and NIHR Clinical Research Network infrastructure support. We acknowledge the generous support of all ISARIC Partners who have contributed data and expertise to this analysis, with or without dedicated funding. The views expressed are those of the authors and not necessarily those of the funders or institutions listed above.
Author information
Authors and Affiliations
Consortia
Contributions
Author contributions are listed in the supplementary material.
Ethics declarations
Conflict of interest
M. Cheng declares grants from McGill Interdisciplinary Initiative in Infection and Immunity, and Canadian Institutes of Health Research; and personal fees from GEn1E Lifesciences (as a member of the scientific advisory board) and nplex biosciences (as a member of the scientific advisory board); M. Cummings and M. O'Donnell participated as investigators for completed and ongoing clinical trials evaluating the efficacy and safety of remdesivir (sponsored by Gilead Sciences) and convalescent plasma (sponsored by Amazon), in hospitalized patients with COVID-19—support for this work is paid to Columbia University; J. C. Holter declared grants from Research Council of Norway [grant 312780], and Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner, during the conduct of the study; A.Kimmoun declared personal fees (payment for lectures) from Baxter, Aguettant, Aspen; D. Kumar declared grants and personal fees from Roche, GSK and Merck, and personal fees from Pfizer and Sanofi; F.X. Lescure declared personal fees (payment for lectures) from Gilead, MSD; and travel/accommodation/meeting expenses from Astellas, Eumedica, MSD; A. Pesenti declared personal fees from Maquet, Novalung/Xenios, Baxter, and Boehringer Ingelheim; S. Shrapnel reported grants from Prince Charles Hospital Foundation during the conduct of the study, and concurrently performed data analytics for the COVID-19 Critical Care Consortium; R. Tedder reports grants from MRC/UKRI during the conduct of the study, and has a patent United Kingdom Patent Application No. 2014047.1 “SARS-CoV-2 antibody detection assay” issued; J. Troost declared personal fees from General Electric and Procter and Gamble.
Ethics approval
This observational study required no change to clinical management and permitted enrolment in other research. The study was approved by the World Health Organization Ethics Review Committee (RPC571 and RPC572). Local ethics approval was obtained for each participating country and site according to local requirements.
Consent to participate
Informed consent was sought where required by local ethics committees. In many jurisdictions, ethics committees and public health authorities approved a waiver of consent to collect anonymised clinical data in the context of this public health emergency.
Consent for publication
Not applicable.
Additional information
Mark G. Pritchard: mark.pritchard@ndm.ox.ac.uk.
Members of the "ISARIC Clinical Characterisation Group" are listed in Acknowledgement section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ISARIC Clinical Characterisation Group. COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study. Infection 49, 889–905 (2021). https://doi.org/10.1007/s15010-021-01599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01599-5