Abstract
Purpose
We investigated the clinical performance of (1 → 3)-β-d-glucan (BG), as an early marker of invasive fungal infections (IFI), in different clinical settings.
Methods
BG serum levels were assessed by Fungitell (Associates of Cape Cod, Inc), in parallel with galactomannan (GM) when requested by clinicians. By a prospective monocentric study, 270 episodes at risk or with suspect of IFI were enrolled, namely 58 proven-probable invasive aspergillosis (IA), 27 proven invasive candidiasis (IC), 11 possible IC, 16 P.jirovecii pneumonia (PJP), 4 episodes of other IFI and 154 non-IFI controls.
Results
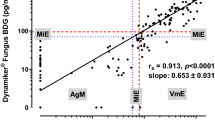
We found that (a) the BG overall sensitivity, specificity, positive predictive value and negative predictive value (NPV) were 87.9, 80.5, 76.7 and 89.9 %, respectively; (b) the highest sensitivity was found in the IC groups, followed by PJP, IA and other IFI groups; (c) an association was observed between BG kinetics and patients outcome; (d) in the IA episodes, the combination of BG or GM vs GM alone increased sensitivity from 60.0 to 83.3 % in the haematological patients; (e) false-positive BG results were related to Gram-negative infections or infusion of polyclonal IgM-enriched immunoglobulins, where high levels of BG were indeed detected.
Conclusion
Besides strengthening its overall good clinical performance, we provide evidence that serum BG correlates with clinical outcome and that, once used in combination with GM, BG allows to enhance IFI diagnosis rate. The high sensitivity and NPV, observed in the Intensive Care Unit setting, open to BG validation as a marker for assessment of antifungal treatment.

Similar content being viewed by others
References
Pittet D, Monod M, Suter PM, et al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751–8.
León C, Ruiz-Santana S, Saavedra P, et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–33. doi:10.1097/CCM.0b013e31819daa14.
Pini P, Bettua C, Orsi CF, et al. Clinical performance of a commercial real-time PCR assay for Aspergillus DNA detection in serum samples from high-risk patients: comparison with a galactomannan enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 2015;34:131–6. doi:10.1007/s10096-014-2211-y.
Orsi CF, Gennari W, Venturelli C, et al. Performance of 2 commercial real-time polymerase chain reaction assays for the detection of Aspergillus and Pneumocystis DNA in bronchoalveolar lavage fluid samples from critical care patients. Diagn Microbiol Infect Dis. 2012. doi:10.1016/j.diagmicrobio.2012.03.001.
Zou M, Tang L, Zhao S, et al. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS ONE. 2012;7:e43347. doi:10.1371/journal.pone.0043347.
Mikulska M, Calandra T, Sanguinetti M, et al. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit Care. 2010;14:222. doi:10.1186/cc9365.
Ardizzoni A, Posteraro B, Baschieri MC, et al. An antibody reactivity-based assay for diagnosis of invasive candidiasis using protein array. Int J Immunopathol Pharmacol. 2014;27:403–12.
Zaragoza R, Pemán J, Quindós G, et al. Kinetic patterns of Candida albicans germ tube antibody in critically ill patients: influence on mortality. Clin Vaccine Immunol. 2009;16:1527–8. doi:10.1128/CVI.00183-09.
De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi:10.1086/588660.
Marchetti O, Lamoth F, Mikulska M, et al. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant. 2012;47:846–54. doi:10.1038/bmt.2011.178.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign. Crit Care Med. 2013;41:580–637. doi:10.1097/CCM.0b013e31827e83af.
Cuenca-Estrella M, Verweij PE, Arendrup MC, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect. 2012;18:9–18. doi:10.1111/1469-0691.12038.
Meersseman W. Invasive Aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–5. doi:10.1164/rccm.200401-093OC.
Meersseman W, Lagrou K, Maertens J, Van Wijngaerden E. Invasive Aspergillosis in the intensive care unit. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;45:205–16. doi:10.1086/518852.
Torelli R, Sanguinetti M, Moody A, et al. Diagnosis of invasive aspergillosis by a commercial real-time PCR assay for Aspergillus DNA in bronchoalveolar lavage fluid samples from high-risk patients compared to a galactomannan enzyme immunoassay. J Clin Microbiol. 2011;49:4273–8. doi:10.1128/JCM.05026-11.
Orsi CF, Bettua C, Pini P, et al. Detection of Pneumocystis jirovecii and Aspergillus spp. DNA in bronchoalveolar lavage fluids by commercial real-time PCR assays: comparison with conventional diagnostic tests. New Microbiol. 2015;38:75–84.
Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1 → 3)-β-d-glucan assay for the diagnosis of invasive fungal infections—a study based on autopsy cases from 6 years. Clin Infect Dis. 2008;46:1864–70. doi:10.1086/588295.
Forstner C, Lassnigg A, Tobudic S, et al. A prospective analysis of invasive candidiasis following cardiac surgery: severity markers are predictive. J Infect. 2013;66:528–35. doi:10.1016/j.jinf.2013.02.003.
Del Bono V, Delfino E, Furfaro E, et al. Clinical performance of the (1 → 3)-β-d-glucan assay in early diagnosis of nosocomial candida bloodstream infections. Clin Vaccine Immunol. 2011;18:2113–7. doi:10.1128/CVI.05408-11.
Hauser PM, Bille J, Lass-Florl C, et al. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jirovecii infection by use of a commercial real-time PCR assay. J Clin Microbiol. 2011;49:1872–8. doi:10.1128/JCM.02390-10.
Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-d-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50:7–15. doi:10.1128/JCM.05267-11.
Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. β-d-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis. 2011;52:750–70. doi:10.1093/cid/ciq206.
He S, Hang J-P, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1, 3-β-d-glucan for invasive fungal infection: focus on cutoff levels. J Microbiol Immunol Infect. 2014. doi:10.1016/j.jmii.2014.06.009.
Lamoth F, Cruciani M, Mengoli C, et al. β-glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of Cohort Studies From the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis. 2012;54:633–43. doi:10.1093/cid/cir89.
Sulahian A, Porcher R, Bergeron A, et al. Use and limits of (1 → 3)-β-d-glucan assay (fungitell), compared to galactomannan determination (platelia Aspergillus), for diagnosis of invasive Aspergillosis. J Clin Microbiol. 2014;52:2328–33. doi:10.1128/JCM.03567-13.
Hachem RY, Kontoyiannis DP, Chemaly RF, et al. Utility of galactomannan enzyme immunoassay and (1,3) β-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J ClinMicrobiol. 2009;47:129–33. doi:10.1128/JCM.00506-08.
Persat F, Ranque S, Derouin F, et al. Contribution of the (1 → 3)-β-d-glucan assay for diagnosis of invasive fungal infections. J ClinMicrobiol. 2008;46:1009–13. doi:10.1128/JCM.02091-07.
Pazos C, Ponton J, Palacio AD. Contribution of (1 → 3)-β-d-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol. 2005;43:299–305. doi:10.1128/JCM.43.1.299-305.2005.
Farina C, Lombardi G, Andreoni S, et al. Routine use of a protease zymogen-based colorimetric assay for the detection of β-glucan and its role in clinical practice. Int J Immunopathol Pharmacol. 2014;27:661–8.
Clancy CJ, Nguyen MH. Finding the “missing 50 %” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56:1284–92. doi:10.1093/cid/cit006.
Hanson KE, Pfeiffer CD, Lease ED, et al. β-d-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study. PLoS ONE. 2012;7:e42282. doi:10.1371/journal.pone.0042282.
Tissot F, Lamoth F, Hauser PM, et al. β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med. 2013;188:1100–9. doi:10.1164/rccm.201211-2069OC.
Mohr JF, Sims C, Paetznick V, et al. Prospective survey of (1 → 3)-β-d-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol. 2010;49:58–61. doi:10.1128/JCM.01240-10.
Posteraro B, De Pascale G, Tumbarello M, et al. Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1 → 3)-β-d-glucan assay, Candida score, and colonization index. Crit Care Lond Engl. 2011;15:R249. doi:10.1186/cc10507.
Sims CR, Jaijakul S, Mohr J, et al. Correlation of clinical outcomes with β-glucan levels in patients with invasive candidiasis. J Clin Microbiol. 2012;50:2104–6. doi:10.1128/JCM.00773-12.
Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. (1,3)-β-d-glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55:521–6. doi:10.1093/cid/cis456.
Lo Cascio G, Koncan R, Stringari G, et al. Interference of confounding factors on the use of (1,3)-β-d-glucan in the diagnosis of invasive candidiasis in the intensive care unit. Eur J ClinMicrobiol Infect Dis. 2014. doi:10.1007/s10096-014-2239-z.
Kedzierska A, Kochan P, Pietrzyk A, Kedzierska J. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1 → 3)-β-d-glucan antigens. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2007;26:755–66. doi:10.1007/s10096-007-0373-6.
Duffner U, Abdel-Mageed A, Dahl K, et al. Serum (1 → 3)-β-d-glucan levels (Fungitell assay) is not useful as a screening test for recipients of an allogeneic HSCT while on immunoglobulin replacement. Bone Marrow Transplant. 2012;47:151–2. doi:10.1038/bmt.2011.24.
Ogawa M, Hori H, Niiguchi S, et al. False-positive plasma (1 → 3)-β-d-d-glucan test following immunoglobulin product replacement in an adult bone marrow recipient. Int J Hematol. 2004;80:97–8. doi:10.1532/IJH97.04030.
Stanzani M, Lewis RE, Fiacchini M, et al. A risk prediction score for invasive mold disease in patients with hematological malignancies. PLoS ONE. 2013;8:e75531. doi:10.1371/journal.pone.0075531.
Bassetti M, Molinari MP, Mussap M, et al. Candidaemia in internal medicine departments: the burden of a rising problem. Clin Microbiol Infect. 2013;19:E281–4. doi:10.1111/1469-0691.12155.
Scudeller L, Viscoli C, Menichetti F, et al. An Italian consensus for invasive candidiasis management (ITALIC). Infection. 2014;42:263–79. doi:10.1007/s15010-013-0558-0.
Acknowledgments
We thank Dr. Andrea Ardizzoni for the critical reading of the manuscript and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in the study, which involved human participants, were in accordance with the ethical standards of the institutional research committee (no. 53/11) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pini, P., Bettua, C., Orsi, C.F. et al. Evaluation of serum (1 → 3)-β-d-glucan clinical performance: kinetic assessment, comparison with galactomannan and evaluation of confounding factors. Infection 44, 223–233 (2016). https://doi.org/10.1007/s15010-015-0849-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-015-0849-8