Abstract
Background:
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is a severe sequela caused by bisphosphonates (BPs), which are widely used to treat osteoporosis or other malignancies. However, the mechanism underlying BRONJ remains unclear. Recently, human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) have been studied for treatment of diverse diseases and injuries. This study aimed to investigate the therapeutic effects of hUC-MSCs in BRONJ.
Methods:
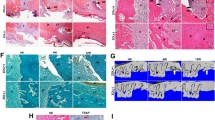
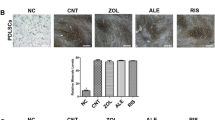
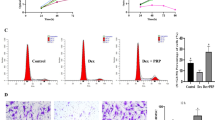
The therapeutic effects of hUC-MSCs were examined in rat bone marrow (rBM)-derived cells using cell viability, colony-forming, and real-time PCR assays and FACS for analyzing essential proinflammatory and bone regeneration markers in vitro. To demonstrate the in vivo therapeutic and adverse effects of transfused hUC-MSCs, micro-CT, H&E staining, IHC (Angiogenesis marker gene expression) staining, and parathyroid hormone (PTH)/calcium assay were conducted in a BRONJ-induced animal model.
Results:
BP-induced cytotoxicity and inflammation in rBM-derived cells decreased, after co-culture with hUC-MSCs. The expression levels of bone regeneration markers (RUNX2, OSX, and BMP-2) significantly increased in BP-treated rBM-derived cells, after co-culture with hUC-MSCs. The BP-induced abnormal shift in RANKL/OPG expression ratio in rBM-derived cells was normalized by hUC-MSCs. Consistent with these in vitro results, transfused hUC-MSCs markedly decreased BRONJ and significantly healed injured mucosa in the BRONJ-induced animal model. The animals exhibited serious destruction of the kidney structure and increases in serum PTH and calcium levels, which were significantly normalized by hUC-MSC transfusion.
Conclusion:
hUC-MSCs exerted therapeutic effects on BRONJ in vitro and in vivo through their anti-cytotoxicity, anti-inflammatory activity and ability to recover bone regeneration.






Similar content being viewed by others
References
Kim J, Lee DH, Dziak R, Ciancio S. Bisphosphonate-related osteonecrosis of the jaw: current clinical significance and treatment strategy review. Am J Dent. 2020;33:115–28.
Silverman SL, Landesberg R. Osteonecrosis of the jaw and the role of bisphosphonates: a critical review. Am J Med. 2009;122:S33–45.
Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): a concise update. Oral Oncol. 2009;45:551–4.
Agrillo A, Filiaci F, Ramieri V, Riccardi E, Quarato D, Rinna C, et al. Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci. 2012;16:1741–7.
Fliefel R, Tröltzsch M, Kühnisch J, Ehrenfeld M, Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg. 2015;44:568–85.
Rosella D, Papi P, Giardino R, Cicalini E, Piccoli L, Pompa G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J Int Soc Prev Community Dent. 2016;6:97–104.
Shin SH, Kim KH, Choi NR, Kim IR, Park BS, Kim YD, et al. Effect of low-level laser therapy on bisphosphonate-treated osteoblasts. Maxillofac Plast Reconstr Surg. 2016;38:48.
Kaibuchi N, Iwata T, Yamato M, Okano T, Ando T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016;42:400–10.
Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92.
Shi LL, Liu FP, Wang DW. Transplantation of human umbilical cord blood mesenchymal stem cells improves survival rates in a rat model of acute hepatic necrosis. Am J Med Sci. 2011;342:212–7.
Zhu H, Xiong Y, Xia Y, Zhang R, Tian D, Wang T, et al. Therapeutic effects of human umbilical cord-derived mesenchymal stem cells in acute lung injury mice. Sci Rep. 2017;7:39889.
Mathieu P, Roca V, Gamba C, Del Pozo A, Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinson’s disease. J Neuroimmunol. 2012;246:43–50.
Rachakatla RS, Pyle MM, Ayuzawa R, Edwards SM, Marini FC, Weiss ML, et al. Combination treatment of human umbilical cord matrix stem cell-based interferon-beta gene therapy and 5-fluorouracil significantly reduces growth of metastatic human breast cancer in SCID mouse lungs. Cancer Invest. 2008;26:662–70.
Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28.
Matsuura Y, Atsuta I, Ayukawa Y, Yamaza T, Kondo R, Takahashi A, et al. Therapeutic interactions between mesenchymal stem cells for healing medication-related osteonecrosis of the jaw. Stem Cell Res Ther. 2016;7:119.
Li Y, et al. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in swine. Stem Cells Dev. 2013;22:2047–56.
Ogata K, Katagiri W, Osugi M, Kawai T, Sugimura Y, Hibi H, et al. Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model. Bone. 2015;74:95–105.
Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem cells. 2008;26:2865–74.
Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNγ and TNFα, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PloS One. 2010;5:e9016.
Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248–69.
Tanna N, Steel C, Stagnell S, Bailey E. Awareness of medication related osteonecrosis of the jaws (MRONJ) amongst general dental practitioners. Br Dent J. 2017;222:121–5.
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938–56.
Ribatti D, Nico B, Mangieri D, Maruotti N, Longo V, Vacca A, et al. Neridronate inhibits angiogenesis in vitro and in vivo. Clin Rheumatol. 2007;26:1094–8.
Wang Y, Long W, Cao Y, Li J, You L, Fan Y. Mesenchymal stem cell-derived secretomes for therapeutic potential of premature infant diseases. Biosci Rep. 2020;40:BSR20200241.
Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8.
He LH, Xiao E, An JG, He Y, Chen S, Zhao L, et al. Role of bone marrow stromal cells in impaired bone repair from BRONJ osseous lesions. J Dent Res. 2017;96:539–46.
Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, Ruiz C, García-Martínez O. High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J Craniomaxillofacial Surg. 2015;43:396–401.
Kossl J, Bohacova P, Hermankova B, Javorkova E, Zajicova A, Holan V. Antiapoptotic Properties of Mesenchymal Stem Cells in a Mouse Model of Corneal Inflammation. Stem Cells Dev. 2021;30:418–427.
Mittal S, Gupta D, Sekhri S, Goyal S. Oral manifestations of parathyroid disorders and its dental management. J Dental Allied Sci. 2014;3:34.
Li JY, D'Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, et al. IL-17A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab. 2015;22:799–810.
Leizaola-Cardesa IO, Aguilar-Salvatierra A, Gonzalez-Jaranay M, Moreu G, Sala-Romero MJ, Gómez-Moreno G. Bisphosphonates, vitamin D, parathyroid hormone, and osteonecrosis of the jaw. Could there be a missing link? Med Oral Patol Oral Cir Bucal. 2016;21:e236–40.
Allam E, Allen M, Chu TM, Ghoneima A, Jack Windsor L. In vivo effects of zoledronic acid on oral mucosal epithelial cells. Oral Dis. 2011;17:291–7.
Kos M, Junka A, Smutnicka D, Szymczyk P, Gluza K, Bartoszewicz M. Bisphosphonates enhance bacterial adhesion and biofilm formation on bone hydroxyapatite. J Craniomaxillofac Surg. 2015;43:863–9.
Kos M, Brusco D, Kuebler J, Engelke W. Clinical comparison of patients with osteonecrosis of the jaws, with and without a history of bisphosphonates administration. Int J Oral Maxillofac Surg. 2010;39:1097–102.
Pettersson LF, Kingham PJ, Wiberg M, Kelk P. In vitro osteogenic differentiation of human mesenchymal stem cells from jawbone compared with dental tissue. Tissue Eng Regen Med. 2017;14:763–74.
Koch FP, Merkel C, Ziebart T, Smeets R, Walter C, Al-Nawas B. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin Oral Investig. 2012;16:79–86.
Hansen T, Kirkpatrick CJ, Walter C, Kunkel M. Increased numbers of osteoclasts expressing cysteine proteinase cathepsin K in patients with infected osteoradionecrosis and bisphosphonate-associated osteonecrosis—a paradoxical observation? Virchows Arch. 2006;449:448–54.
Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29:555–67.
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29.
Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3:131–45.
Sun J, Li J, Li C, Yu Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol Med Rep. 2015;12:4230–7.
Zhang Q, Yu W, Lee S, Xu Q, Naji A, Le AD. Bisphosphonate induces osteonecrosis of the jaw in diabetic mice via NLRP3/Caspase-1-dependent IL-1β mechanism. J Bone Miner Res. 2015;30:2300–12.
Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, et al. Interleukin-6 and tumor necrosis factor α levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–9.
Melcher M, Danhauser K, Seibt A, Degistirici Ö, Baertling F, Kondadi AK, et al. Modulation of oxidative phosphorylation and redox homeostasis in mitochondrial NDUFS4 deficiency via mesenchymal stem cells. Stem Cell Res Ther. 2017;8:150.
Acknowledgements
We would like to thank Asan stem cell center for providing hUC-MSCs. We also thank Professor Woo-Chan Son for the assistance with histopathologic evaluation, Asan Medical Center, for the use of their shared equipment, services, and expertise. This study was supported by a Grant (No. 2016-569) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea. This study was supported by a grant (2018-327) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Author information
Authors and Affiliations
Contributions
YNK and GY was responsible for the analysis and interpretation of the data, as well as drafting of the article. HK contributed to the study performance. BKL is the corresponding author, responsible for conception and design, as well as critical revision of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) Asan Institute for Life Sciences, Asan Medical Center (NO: 2017-12-208). The committee abides by the Institute of Laboratory Animal Resources (ILAR) guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, G., Kim, YN., Kim, H. et al. Effect of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells on Bisphosphonate-Related Osteonecrosis of the Jaw. Tissue Eng Regen Med 18, 975–988 (2021). https://doi.org/10.1007/s13770-021-00372-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00372-x